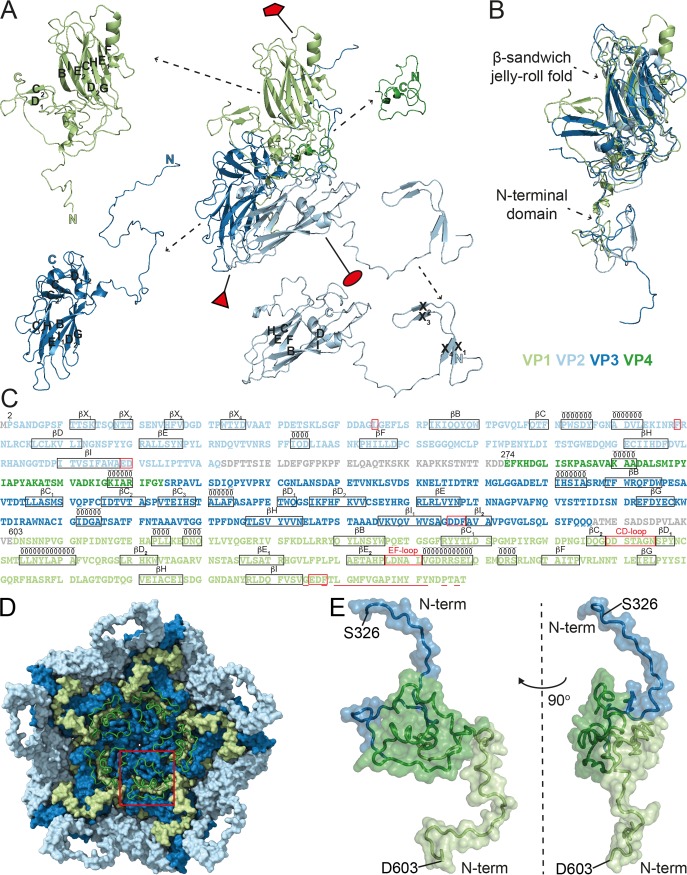
FIG 3.
Structures and the amino acid sequences of the CtenRNAV-II CPs. The individual proteins are colored according to the following code: VP1, light green; VP2, light blue; VP3, dark blue; VP4, dark green. (A) The secondary structure for one single icosahedral protomer. The positions of the five-, three-, and two-fold axes are shown by a red pentamer, triangle, and ellipse, respectively. The β-strands within the β-sheets are named alphabetically according to the conventional jelly-roll fold nomenclature (B to I). (B) Superimposition of the three major CPs, VP1-3, exhibits the β-sandwich jelly-roll folds and N-terminal extensions in all three CPs. (C) The amino acid sequences of the CtenRNAV-II CPs. The proteins are listed in the order in which they appear in the genome, and the residue numbering follows this order, which is the same as in PDB entry 6SHL. The number of the first residue that was modeled in each protein is written out above those residues. Each line has 100 residues and is further subdivided into blocks of 10 residues by spaces within the sequence. Unobserved regions of the proteins are shown in gray text. The assigned secondary structure is shown schematically above the sequence. Areas discussed in this paper are highlighted in red: L54 and F99 of VP2 that might be of importance for the N-terminal domain swap; the EDF and DDF proteolytic motifs following strand βI in VP1 and VP3, respectively, as well as the ED residues at the corresponding position in VP2; the CD- and EF-loop in VP1; and the residues considered hydrophobic in the N terminus of VP1 and discussed in regard to cell entry are underlined. (D) A pentamer of CP protomers as seen from the inside looking down the five-fold axis. The VP1-3 proteins are rendered with a surface representation, and the VP4 protein is rendered with a ribbon representation. The red square highlights the position of one VP4 molecule. The N terminus of VP4 is located toward the five-fold axis. (E) The position of VP4 in relation to VP1 and VP3. The figure shows the N-terminal domains of VP3 (S326 to D367) and VP1 (D603 to R648) in the top and bottom of the picture, respectively. Left: same view as in (D), i.e., looking at the inside of the capsid; right: rotated 90° to the left.