Abstract
Objective:
The use of the radial approach in coronary angiography or percutaneous coronary intervention has increased owing to its advantages over the femoral approach such as rapid patient mobilization and improved patient comfort. However, radial artery spasm (RAS) that occurs during the procedure is a crucial factor in transradial approach failure and access site switch. Asymmetric dimethylarginine (ADMA) is a naturally occurring, modified amino acid that inhibits nitric oxide (NO) production. High ADMA levels may reduce arterial elasticity especially in small arteries like the radial artery. This study aimed to evaluate the relationship between ADMA levels and RAS in radial artery access.
Methods:
This study included 155 patients (89 males and 66 females) who underwent transradial coronary angiography between January 2016 and June 2016. The ADMA level in the plasma was determined using a quantitative sandwich enzyme immunoassay technique.
Results:
RAS was observed in 16 of the 155 patients (10.1%). The RAS was found to be more frequent in female patients (17.9% for women vs. 4.4% for men, p=0.019). The plasma concentration of ADMA in the RAS group was significantly higher than that in the control group [22.1 ng/mL (12.1–37.8) vs. 9.2 ng/mL (5.9–14.8), p<0.001]. Moreover, the plasma concentration of ADMA was significantly higher in patients with RAS among female patients [20.4 ng/mL (12.1–44.9) vs. 9.9 ng/mL (6.2–16.6); p=0.002] and among male patients [25.2 ng/mL (13.7–35.4) vs. 8.2 ng/mL (5.9–12.8); p=0.007]. Binary logistic regression analysis of all patients showed that ADMA concentration was the only predictor for RAS (odds ratio=1.142; 95% confidence interval=1.061–1.228; p<0.001).
Conclusion:
It was found that the ADMA concentration of the patients in the RAS group was elevated compared to that of controls. The findings indicated that elevated ADMA concentrations could predict RAS that may occur. (Anatol J Cardiol 2020; 23: 228-32)
Keywords: radial artery, vasospasm, asymmetric dimethylarginine
Introduction
The use of the radial approach in coronary angiography or percutaneous coronary intervention has increased owing to its advantages such as rapid patient mobilization and improved patient comfort. Compared to the femoral approach, transradial artery access is associated with lower bleeding and vascular complications and reduced length of hospital stay (1-4). The radial artery is the preferred entry site especially for patients who are at increased risk for bleeding and require intensive antithrombotic therapies. The ulnar collateral circulation maintains the blood supply of the hand if the radial artery is occluded; hence, the risk of distal embolic and ischemic complications is very low. The transradial approach is becoming more popular for noncoronary interventions such as cerebral and renal interventions. However, radial artery spasm (RAS) that occurs during the procedure is a crucial factor in transradial approach failure and access site switch (5).
The radial artery is composed mainly of smooth muscle cells in concentric layers, making it more susceptible to spasm compared to other arteries. This marked muscular component and increased density of alpha-1 receptors make the radial artery more prone to spasms (6). RAS is a common complication encountered by radial operators during transradial interventions which causes patient discomfort and reduces the chance of a successful catheterization. Even in competent centers, radial spasm occurs in 15%–30% of the procedures (7). This implies the need for risk factor determination before the transradial access. Several clinical and anatomical factors have been identified as risk factors for RAS, namely, female sex, younger age, lower body mass index, smaller radial artery diameter, presence of atherosclerotic lesions, vessel tortuosity, entrance of guide wires into side branches, and larger arterial sheath diameter (8, 9).
Asymmetric dimethylarginine (ADMA) is a naturally occurring, modified amino acid which inhibits nitric oxide (NO) production. High ADMA levels may cause endothelial vasodilatory dysfunction and reduced arterial elasticity especially in small arteries like the radial artery. This study aimed to evaluate the relationship between ADMA levels and RAS in radial artery access. To our knowledge, this study is the first to investigate the relationship.
Methods
This prospective study was conducted in the Department of Cardiology, Sakarya University School of Medicine, Sakarya, Turkey, between January 2016 and June 2016. This study consecutively included 155 patients with unstable angina or angina resistant to medical therapy who were planned for transradial coronary angiography. Patients who had abnormal Allen’s test findings and those with acute myocardial infarction were excluded from the study. An average of 4.000 coronary angiographies or interventions have been performed in our tertiary center each year and we have experienced radial operators. Radial access in coronary interventions was applied in >70% of patients in our center, and all procedures were performed by experienced radial operators (radial approach applied in > 50% of their cases). An informed written consent was obtained from the participants and Local Ethical Committee approval was obtained before the study was started.
Laboratory analysis
Blood samples were centrifuged immediately after collection at 3.000 rpm for 10 minutes at 4°C. The supernatant was then kept frozen at −80°C until it was analyzed. The ADMA level in the plasma was determined through a quantitative sandwich enzyme immunoassay technique using a commercially available kit (CUSABIO Human asymmetrical Dimethylarginine ELISA Kit, catalog number CSB-E09298h, CUSABIO Technology LLC, Houston, TX 77036, USA) on a BioTekGen5 ELx800device (BioTek Instruments, Inc., Winooski, USA).
Interventional technique
Patients
The arterial puncture was carried out using a 5F puncture set (Prelude Sheath, Utah, USA) immediately after anesthetizing the access site with a local anesthetic (prilocaine 2%). Verapamil at 200 mg and heparin at 3000 U were given prophylactically along the sheath to prevent RAS and thromboembolic events. The sheath was removed after the procedure and the puncture site was compressed to achieve hemostasis. The compression was released slowly and the transradial bandage was fully deflated after 4–6 hours.
Clinical definition of radial artery spasm
The operator assessed the RAS using a questionnaire addressing five signs: persistent forearm pain, pain response on catheter manipulation, pain response on introducer withdrawal, difficult manipulation of the catheter due to the entrapment by RAS, and considerable resistance on withdrawal of the introducer. RAS was clinically diagnosed by the presence of at least two of the five signs (8).
Statistical analysis
Parameters were expressed as mean±SD in normal distribution, and parameters with abnormal distribution were expressed as median of the 25th–75th percentile. The chi-square and the Student’s t-test were used for categorical and continuous variables, respectively. Fisher’s exact test was applied in analyzing small samples. A p-value less than 0.05 was considered statistically significant. Statistical analyses were performed using statistical software (SPSS 20.0, Chicago, IL, USA). For continuous variables, differences between the two groups were evaluated using the Student’s t-test when data were normally distributed and the Mann–Whitney U test when the assumption of normality was not met. Binary logistic regression analysis was performed to determine independent factors associated with RAS.
Results
This study included 155 consecutive patients (89 males and 66 females) who underwent coronary angiography via radial access between January 2016 and June 2016. Patients with acute myocardial infarction were excluded from the study, and all the patients had an indication for coronary angiography such as unstable angina and angina resistant to medical therapy. The mean age was 62.8±9.4 years in patients with RAS and 60.2±11.5 years in patients without RAS (p=0.381). Patient age and history of hypertension, diabetes, hypercholesterolemia, previous coronary artery disease, peripheral artery disease, and smoking incidence were similar in the two groups. Serum creatine, low-density lipoprotein cholesterol, hemoglobin, and glucose levels were also similar in the two groups. Radial artery spasm was observed in 16 (10%) of the 159 patients, and RAS was more frequent in female patients (18.1% for women vs. 4.5% for men, p=0.019). Radial artery occlusion was observed in one patient from the RAS group and in three from the control group (6.2% vs. 2.1%, p=0.328). Table 1 presents the demographic characteristics and the laboratory findings of the groups. The plasma concentration of ADMA in the RAS group was significantly higher than that in the control group [22.1 ng/mL (12.1–37.8) vs. 9.2 ng/mL (5.9–14.8), p<0.001]. Figure 1 shows the plasma concentrations of ADMA in the RAS and control groups. The plasma concentration of ADMA was significantly higher in patients with RAS among female patients [20.4 ng/mL (12.1–44.9) vs. 9.9 ng/mL (6.2–16.6); p=0.002] and among male patients [25.2 ng/mL (13.7–35.4) vs. 8.2 ng/mL (5.9–12.8); p=0.007]. The area under the receiver operating characteristic curve for ADMA of all patients was 0.836±0.05 (p<0.001). This result suggested that ADMA levels have a direct relation to RAS. When the cut-off value of ADMA was accepted as 10.5 ng/mL, the specificity and sensitivity were 61.2% and 87.5%, respectively. Contrarily, when 19.6 ng/mL was used as the cut-off point, the specificity and sensitivity were 84.9% and 62.5%, respectively. Binary logistic regression analysis was performed to identify independent factors associated with RAS. Age, gender, smoking, hypertension, diabetes mellitus, and ADMA concentration were included in the equation. ADMA concentration was found to be the only predictor for RAS (odds ratio=1.142, 95% confidence interval=1.061–1.228, p<0.001) (Table 2).
Table 1.
Baseline characteristics and laboratory findings of radial artery spasm group and controls
| RAS (+) (n=16) | RAS (–) (n=143) | P | |
|---|---|---|---|
| Age (years) | 62.8±9.4 | 60.2±11.5 | 0.381 |
| Male sex | 4 (25.0%) | 85 (61.1%) | 0.006 |
| Diabetes mellitus | 6 (37.5%) | 52 (37.4%) | 0.994 |
| Hypertension | 10 (62.5%) | 67 (48.2%) | 0.279 |
| Dyslipidemia | 1 (6.2%) | 17 (12.2%) | 0.696 |
| Coronary artery disease | 1 (6.2%) | 26 (18.7%) | 0.309 |
| Peripheral artery disease | 1 (6.2%) | 13 (9.3%) | 0.682 |
| Smoking | 7 (43.7%) | 63 (45.3%) | 0.905 |
| Hemoglobin (g/dL) | 13.3±1.6 | 13.4±1.6 | 0.766 |
| Creatine (mg/dL) | 0.83±0.21 | 0.84±0.19 | 0.917 |
| LDL cholesterol (mg/dL) | 122.4±30.2 | 121.6±36.4 | 0.935 |
| Blood glucose (mg/dL) | 122.4±50.6 | 124.6±56.8 | 0.882 |
Data are presented as mean±SD or number (percentage). RAS - radial artery spasm; LDL - low-density lipoprotein
Figure 1.
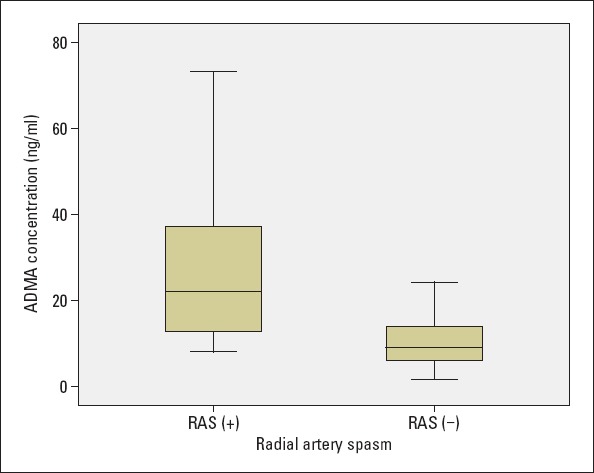
ADMA concentrations in RAS group and control group. ADMA concentrations were significantly higher in the radial artery spasm group (p<0.001)
ADMA - asymmetric dimethylarginine; RAS - radial artery spasm
Table 2.
Independent factors associated with radial artery spasm using binary logistic regression analysis
| Variables | Exp (B) (odds ratio) | 95% confidence interval | P |
|---|---|---|---|
| Age | 1.037 | 0.974-1.103 | 0.255 |
| Female gender | 2.727 | 0.694-10.712 | 0.151 |
| Smoking | 0.918 | 0.227-3.710 | 0.904 |
| Hypertension | 0.811 | 0.217-3.033 | 0.755 |
| Diabetes mellitus | 0.912 | 0.227-3.660 | 0.896 |
| ADMA concentrations | 1.142 | 1.061-1.228 | <0.001 |
ADMA - asymmetric dimethylarginine
Discussion
In this study, it was found that the plasma concentration of ADMA was significantly higher in the RAS group. ADMA is an endogenous competitive inhibitor of NO synthase. NO has an important role in arterial elasticity and endothelial function. It has been reported that increased ADMA levels are associated with atherosclerosis and future cardiovascular events (10). Previous studies noted elevated plasma ADMA levels in patients with coronary artery disease, peripheral arterial disease, chronic heart failure, diabetes mellitus, and chronic renal failure (10-15). Furthermore, the relationship between ADMA levels and arterial stiffness was also reported (16). The association between RAS during coronary angiography and plasma ADMA levels has not been investigated previously. This study demonstrated the association of serum ADMA and RAS for the first time in the literature. It was considered that ADMA accumulation may increase the risk of RAS due to deterioration in the release of NO.
NO is an endothelium-derived vasoactive substance and plays an important role in the regulation of vascular tonus, platelet activity, and the development of atherosclerosis. NO is a potent vasodilator and decreased nitric oxide levels are associated with endothelial dysfunction. It is synthesized from L-arginine through the action of NO synthase. ADMA is an endogenous competitive inhibitor of NO synthase. Several reports showed the association between arterial elasticity and endothelial function, most likely relating to release of NO. Some previous reports also showed that small artery elasticity was inversely associated with ADMA (16-19). In this study, ADMA levels were found to be elevated in patients with RAS. The findings of this study were consistent with those of previous studies in the literature.
Radial artery access has been increasingly used for percutaneous coronary procedures. The advantages of transradial access are improved patient comfort, early mobilization, decreased bleeding complications, and reduced in-hospital stay (1-4). However, radial artery vasospasm remains as a crucial concern. The radial artery has a relatively small diameter and is abundant in smooth muscles, making it more susceptible to spasm than other arterial pathways. Furthermore, dysregulation of serotonin and NO levels triggered by endothelial dysfunction may contribute to vasospasm. This study suggested that higher ADMA levels is a significant risk factor for RAS possibly decreasing by NO levels.
Elevated ADMA levels has recently emerged as a novel cardiovascular risk factor (20). ADMA accumulation may cause endothelial vasodilatory dysfunction and reduced arterial elasticity particularly in small arteries. It has been shown that infusions of ADMA decreases renal plasma flow and increases pulmonary vascular resistance (21, 22). Furthermore, elevated ADMA levels were noted in patients with left ventricular hypertrophy (23). These findings may explain the relationship between ADMA and arterial stiffness in large arteries as well.
Endothelium-derived nitric oxide and the most important inhibitor of nitric oxide, ADMA, have a significant effect on the tonus of arteries such as the radial artery. However, there is no study in the literature that clearly demonstrates this relationship. This study suggested that increased vascular tone responsible for RAS is due to increased ADMA levels. This effect may be increased especially in small arteries. Revealing this relationship may facilitate early detection and further prevention inpatients prone to RAS.
Study limitations
The study limitations included the study being conducted in a single center and the relatively small sample size. This was a pilot study and larger-scale multicenter studies are warranted to confirm the relationship between ADMA and RAS.
A critical limitation of this study was that the radial artery diameter, an important risk factor for RAS, was not evaluated. Female patients have a smaller radial artery compared to males, which might explain why females have a high risk for vasospasm. In this study, there was no certain method used in measuring the radial artery spasm, and RAS was determined based on clinical signs and findings.
Conclusion
This study demonstrated that elevated ADMA levels were associated with radial spasm in patients who underwent transradial angiography. Future large prospective studies are required to determine whether ADMA levels affect radial vasospasm.
Footnotes
Conflict of interest: None declared.
Peer-review: Externally peer-reviewed.
Authorship contributions: Concept – M.A.Ç., E.T.; Design – M.A.Ç.; Supervision – E.T., R.A.; Funding – İ.K., B.K., M.N.M.A.; Materials – B.K., M.N.M.A.; Data collection and/or processing – İ.K., M.A.Ç., B.K.; Analysis and/or interpretation – İ.K., M.N.M.A.; Literature search – İ.K.; Writing – İ.K., R.A; Critical review – E.T., R.A.
References
- 1.Feldman DN, Swaminathan RV, Kaltenbach LA, Baklanov DV, Kim LK, Wong SC, et al. Adoption of radial access and comparison of outcomes to femoral access in percutaneous coronary intervention:an updated report from the national cardiovascular data registry (2007-2012) Circulation. 2013;127:2295–306. doi: 10.1161/CIRCULATIONAHA.112.000536. [DOI] [PubMed] [Google Scholar]
- 2.Ferrante G, Rao SV, Jüni P, Da Costa BR, Reimers B, Condorelli G, et al. Radial Versus Femoral Access for Coronary Interventions Across the Entire Spectrum of Patients With Coronary Artery Disease:A Meta-Analysis of Randomized Trials. JACC Cardiovasc Interv. 2016;9:1419–34. doi: 10.1016/j.jcin.2016.04.014. [DOI] [PubMed] [Google Scholar]
- 3.Mann T, Cubeddu G, Bowen J, Schneider JE, Arrowood M, Newman WN, et al. Stenting in acute coronary syndromes:a comparison of radial versus femoral access sites. J Am Coll Cardiol. 1998;32:572–6. doi: 10.1016/s0735-1097(98)00288-5. [DOI] [PubMed] [Google Scholar]
- 4.Cooper CJ, El-Shiekh RA, Cohen DJ, Blaesing L, Burket MW, Basu A, et al. Effect of transradial access on quality of life and cost of cardiac catheterization:A randomized comparison. Am Heart J. 1999;138(3 Pt 1):430–6. doi: 10.1016/s0002-8703(99)70143-2. [DOI] [PubMed] [Google Scholar]
- 5.Abdelaal E, Brousseau-Provencher C, Montminy S, Plourde G, MacHaalany J, Bataille Y, et al. Interventional Cardiologists at Quebec Heart-Lung Institute. Risk score, causes, and clinical impact of failure of transradial approach for percutaneous coronary interventions. JACC Cardiovasc Interv. 2013;6:1129–37. doi: 10.1016/j.jcin.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 6.He GW, Yang CQ. Characteristics of adrenoreceptors in the human radial artery:clinical implications. J Thorac Cardiovasc Surg. 1998;115:1136–41. doi: 10.1016/S0022-5223(98)70414-3. [DOI] [PubMed] [Google Scholar]
- 7.Kiemeneij F, Vajifdar BU, Eccleshall SC, Laarman G, Slagboom T, van der Wieken R. Evaluation of a spasmolytic cocktail to prevent radial artery spasm during coronary procedures. Catheter Cardiovasc Interv. 2003;58:281–4. doi: 10.1002/ccd.10445. [DOI] [PubMed] [Google Scholar]
- 8.Jia DA, Zhou YJ, Shi DM, Liu YY, Wang JL, Liu XL, et al. Incidence and predictors of radial artery spasm during transradial coronary angiography and intervention. Chin Med J. 2010;123:843–7. [PubMed] [Google Scholar]
- 9.Fukuda N, Iwahara S, Harada A, Yokoyama S, Akutsu K, Takano M, et al. Vasospasms of the radial artery after the transradial approach for coronary angiography and angioplasty. Jpn Heart J. 2004;45:723–31. doi: 10.1536/jhj.45.723. [DOI] [PubMed] [Google Scholar]
- 10.Sibal L, Agarwal SC, Home PD, Boger RH. The Role of Asymmetric Dimethylarginine (ADMA) in Endothelial Dysfunction and Cardiovascular Disease. Curr Cardiol Rev. 2010;6:82–90. doi: 10.2174/157340310791162659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Valkonen VP, Päivä H, Salonen JT, Lakka TA, Lehtimäki T, Laakso J, et al. Risk of acute coronary events and serum concentration of asymmetrical dimethylarginine. Lancet. 2001;358:2127–8. doi: 10.1016/S0140-6736(01)07184-7. [DOI] [PubMed] [Google Scholar]
- 12.Böger RH, Maas R, Schulze F, Schwedhelm E. Asymmetric dimethylarginine (ADMA) as a prospective marker of cardiovascular disease and mortality--an update on patient populations with a wide range of cardiovascular risk. Pharmacol Res. 2009;60:481–7. doi: 10.1016/j.phrs.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 13.Böger RH, Bode-Böger SM, Thiele W, Junker W, Alexander K, Frölich JC. Biochemical evidence for impaired nitric oxide synthesis in patients with peripheral arterial occlusive disease. Circulation. 1997;95:2068–74. doi: 10.1161/01.cir.95.8.2068. [DOI] [PubMed] [Google Scholar]
- 14.Usui M, Matsuoka H, Miyazaki H, Ueda S, Okuda S, Imaizumi T. Increased endogenous nitric oxide synthase inhibitor in patients with congestive heart failure. Life Sci. 1998;62:2425–30. doi: 10.1016/s0024-3205(98)00225-2. [DOI] [PubMed] [Google Scholar]
- 15.Dimitrow PP, Undas A, Bober M, Tracz W, Dubiel JS. Plasma biomarkers of endothelial dysfunction in patients with hypertrophic cardiomyopathy. Pharmacol Rep. 2007;59:715–20. [PubMed] [Google Scholar]
- 16.Kals J, Kampus P, Kals M, Teesalu R, Zilmer K, Pulges A, et al. Arterial elasticity is associated with endothelial vasodilatory function and asymmetric dimethylarginine level in healthy subjects. Scand J Clin Lab Invest. 2007;67:536–44. doi: 10.1080/00365510701203470. [DOI] [PubMed] [Google Scholar]
- 17.Wilson AM, O'Neal D, Nelson CL, Prior DL, Best JD, Jenkins AJ. Comparison of arterial assessments in low and high vascular disease risk groups. Am J Hypertens. 2004;17:285–91. doi: 10.1016/j.amjhyper.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 18.Parvathaneni L, Harp J, Zelinger A, Silver MA. Relation between brachial artery reactivity and noninvasive large and small arterial compliance in healthy volunteers. Am J Cardiol. 2002;89:894–5. doi: 10.1016/s0002-9149(02)02212-9. [DOI] [PubMed] [Google Scholar]
- 19.Tao J, Jin YF, Yang Z, Wang LC, Gao XR, Lui L, et al. Reduced arterial elasticity is associated with endothelial dysfunction in persons of advancing age:comparative study of noninvasive pulse wave analysis and laser Doppler blood flow measurement. Am J Hypertens. 2004;17:654–9. doi: 10.1016/j.amjhyper.2004.03.678. [DOI] [PubMed] [Google Scholar]
- 20.Cooke JP. Asymmetrical dimethylarginine:the Uber marker?Circulation. 2004;109:1813–8. doi: 10.1161/01.CIR.0000126823.07732.D5. [DOI] [PubMed] [Google Scholar]
- 21.Kielstein JT, Impraim B, Simmel S, Bode-Böger SM, Tsikas D, Frölich JC, et al. Cardiovascular effects of systemic nitric oxide synthase inhibition with asymmetrical dimethylarginine in humans. Circulation. 2004;109:172–7. doi: 10.1161/01.CIR.0000105764.22626.B1. [DOI] [PubMed] [Google Scholar]
- 22.Kielstein JT, Bode-Böger SM, Hesse G, Martens-Lobenhoffer J, Takacs A, Fliser D, et al. Asymmetrical dimethylarginine in idiopathic pulmonary arterial hypertension. Arterioscler Thromb Vasc Biol. 2005;25:1414–8. doi: 10.1161/01.ATV.0000168414.06853.f0. [DOI] [PubMed] [Google Scholar]
- 23.Zoccali C, Mallamaci F, Maas R, Benedetto FA, Tripepi G, Malatino LS, et al. CREED Investigators Left ventricular hypertrophy, cardiac remodeling and asymmetric dimethylarginine (ADMA) in hemodialysis patients. Kidney Int. 2002;62:339–45. doi: 10.1046/j.1523-1755.2002.00437.x. [DOI] [PubMed] [Google Scholar]


