FIG 3.
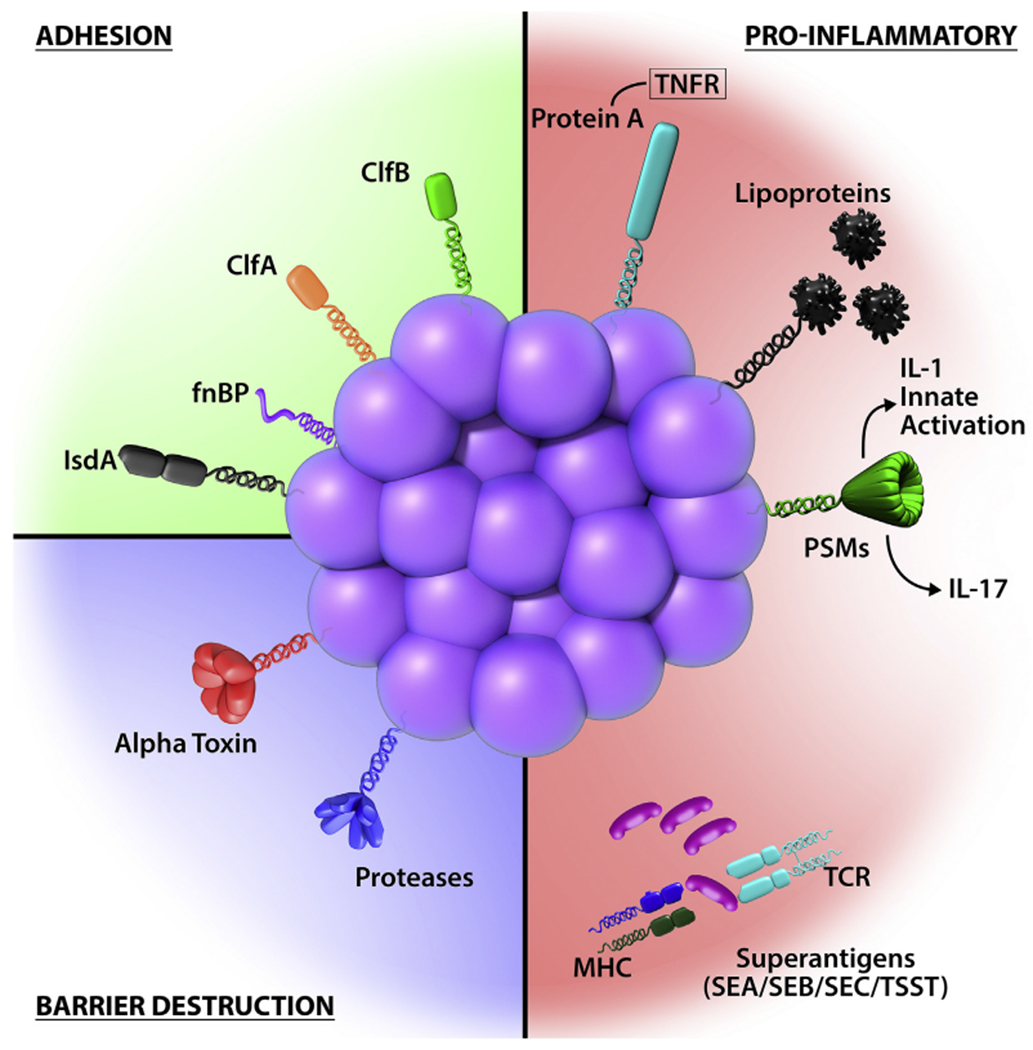
S aureus has highly evolved multiple cell-wall proteins and secreted factors that enable adhesion to human skin and barrier disturbance by using physical, chemical, and inflammatory mechanisms. Adhesion, S aureus has developed several surface molecules to adhere to the human stratum corneum, including clumping factors A and B (ClfA and ClfB), fibronectin-binding protein (fnBP), and iron-regulated surface determinant A (IsdA). Barrier destruction, S aureus α-toxin, a water-soluble cytotoxin, forms a heptameric β-barrel pore in host cell membranes. In the epidermis it directlyforms pores in keratinocytes, which erodes the integrity of the epidermal barrier. S aureus produces at least 10 proteases, a number of which facilitate dissolution of the stratum corneum. In addition to secreted proteases, S aureus can directly stimulate endogenous keratinocyte proteases, including KLK6, KLK13, and KLK14, highlighting an additional mechanism toward barrier destruction. Proinflammatory mechanisms, Cell-wall bound protein A, when solubilized, triggers inflammatory responses from keratinocytes through TNF receptor (TNFR). Staphylococcal superantigens, such as SEA, SEB, SEC, and toxic shock syndrome toxin-1 (TSST-1), trigger B-cell expansion and cytokine release. S aureus secretes PSMs, which are direct proinflammatory drivers with compartment-specific effects. In the epidermal compartment PSMs stimulate IL-36α-driven γδ T cell–mediated inflammation, whereasinthe dermal compartmentthey stimulate IL-1β-driven TH17 inflammation.
