Figure 4.
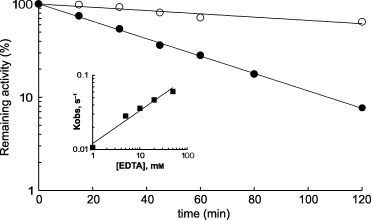
Inactivation of A. pisum APN by EDTA at 37 °C. Reaction mixtures contained different concentrations of EDTA in 100 mm Tris/HCl buffer, pH 7.0, containing 0.1% Triton X‐100. After different incubation times, the reaction was stopped by 100 times dilution. Inactivation by 50 mm EDTA in the absence (•) or presence (○) of 850 µm (25 × K i) arginine hydroxamate, which is a competitive inhibitor of aminopeptidase. Buffer used: 100 mm Tris/HCl, pH 7.0, containing 0.1% Triton X‐100. The insert shows a plot of the log of the observed first‐order rate kinetics of inactivation constant against log of EDTA concentration. n, the slope of the plot, was calculated as 0.44 and estimates the number of molecules of EDTA needed to inactivate each active site of the enzyme.
