Figure 3.
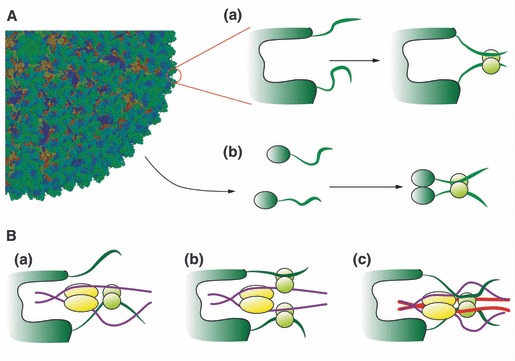
(A) Model for the interaction of a generic viral capsid with cytoplasmic, non‐microtubule‐associated DYNLL. It is then conceivable that the DYNLL homodimer might bind simultaneously to two viral polypeptides when part of the viral capsid (a) or when soluble after viral disassembly (b). This is in agreement with the modeled solution structure of the complex of DYNLL1 with p54 of ASFV [26]. (B) Three hypotheses for the association of viral proteins to the dynein molecular motor. One viral polypeptide displaces a dynein intermediate chain from one binding side of the DYNLL homodimer (a). Two DYNLL homodimers associate to one dynein intermediate chain and to one viral polypeptide simultaneously (b). Binding of the viral polypeptides displaces the dynein intermediate chains from the DYNLL binding grooves but DYNLL remains part of the dynein motor through the binding to the dynein heavy chain (red) (c).
