Figure 6.
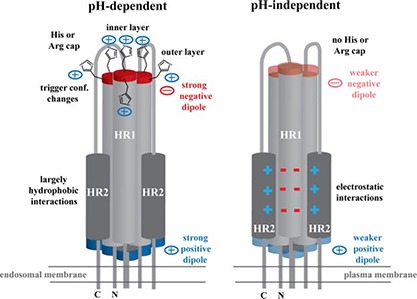
Summary of pH‐dependent and pH‐independent fusion protein properties. Cartoon shows the viral fusion subunit in the postfusion conformation. Viruses that require low pH for entry, such as ASLV, IAV, and LCMV, fuse at the endosomal membrane and have strong positive and negative charges at their ends due to its helix dipole moment. Negatively charged helix dipole is stabilized by two layers of positively charged histidine or arginine capping residues (only histidine is shown). In addition, the outer layer of histidine residues may be involved in triggering conformational changes in the prefusion viral glycoprotein on entering a low‐pH environment. HR1‐HR2 interface is in general mediated by largely hydrophobic interactions. Viruses that undergo a pH‐independent entry process, such as retroviruses, fuse at the plasma membrane. The helix dipole moment is decreased, and no histidine or arginine residues are located in the CR region to stabilize the negative helix dipole. Moreover, the HR1‐HR2 interface is stabilized with a larger number of electrostatic interactions rather than hydrophobic forces.
