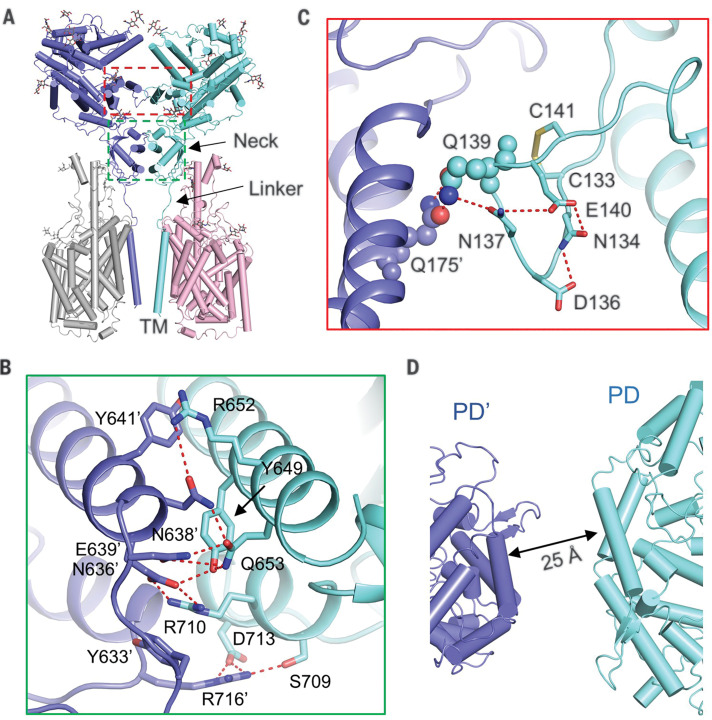
Fig. 2. Dimerization interface of ACE2.
(A) ACE2 dimerizes through two interfaces, the PD and the neck domain. The regions enclosed by the cyan and red dashed lines are illustrated in detail in (B) and (C), respectively. (B) The primary dimeric interface is through the neck domain in ACE2. Polar interactions are represented by red dashed lines. (C) A weaker interface between PDs of ACE2. The only interaction is between Gln139 and Gln175′, which are highlighted as spheres. The polar residues that may contribute to the stabilization of Gln139 are shown as sticks. (D) The PDs no longer contact each other in the open state. Single-letter abbreviations for the amino acid residues used in the figures are as follows: C, Cys; D, Asp; E, Glu; F, Phe; H, His; K, Lys; L, Leu; M, Met; N, Asn; Q, Gln; R, Arg; S, Ser; T, Thr; V, Val; and Y, Tyr.