Table 1.
Generation of Aza-Anthraquinones via Photoelectrocyclization Reactions
 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| entry | X | Conditions | Coupling Yield | Oxidation Yield | aminoquinone | time (min.)b | quinoline | yield | quinolone | yield |
| 1 | CI | A | 76% | 75% | 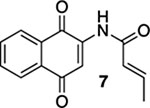 |
255 | 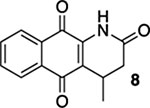 |
47% | 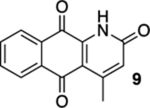 |
50% |
| 2 | OH | B | 56% | 80% | 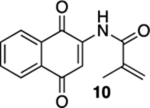 |
75 | 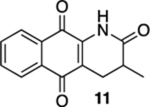 |
76% | 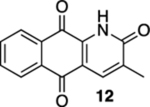 |
21% |
| 3 | OH | B | 81% | 95% | 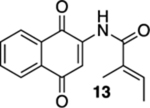 |
25 | 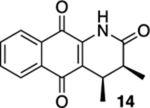 |
90% (cis:trans = 15:1) | 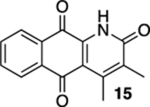 |
--d |
| 4 | CI | A | 78% | 70% | 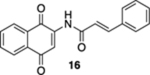 |
50 | 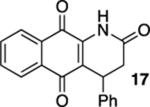 |
33% | 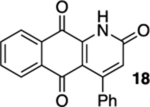 |
--e |
| 5 | OH | B | — | 52%c | 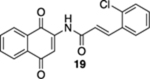 |
60 | 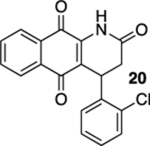 |
20% |  |
--e |
| 6 | OH | B | — | 45%c | 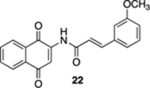 |
60 | 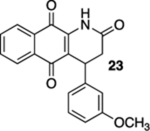 |
20% | 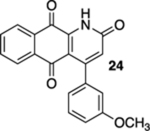 |
--e |
Conditions: A: Et3N, CH2Cl2; B: EDC·HCl, 4-DMAP, and CH2Cl2.
Time required for the disappearance of the starting material by thin-layer chromatography (TLC).
Yield for two steps.
A trace amount of 15 was formed if the reaction was allowed to proceed for more than 60 min.
An intractable mixture of what we presume as oxidized byproducts was obtained.
