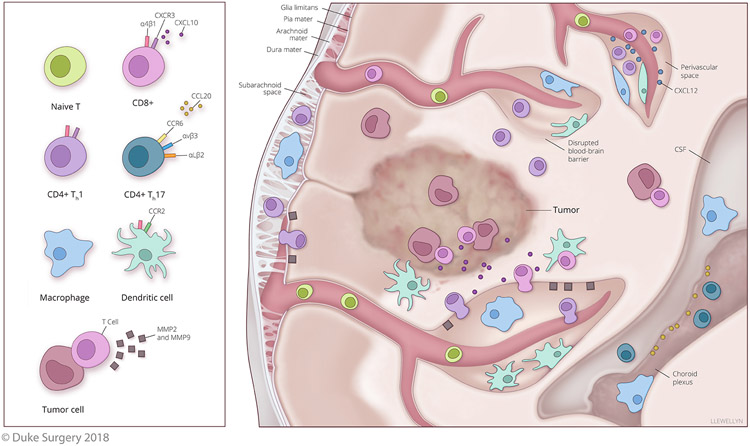
Figure 1. Proposed Mechanisms of T cell Infiltration of the CNS and the Generation of Antitumor Immunity.
Evidence indicates that activated T cells expressing CXCR3 (potentially recruited via CNS expression of CXCL10) will preferentially traffic to the CNS. Infiltration of the CNS has also been shown to be dependent on receptor expression and T cell subtype. Th1 CD4+ and CD8+ T cells show a dependence on α4β1 integrin for CNS infiltration, whereas Th17 T cells show a dependence on the integrins αVβ3 and αLβ2 as well as the chemokine receptor CCR6 binding to CCL20 for infiltration of the CSF from the choroid plexus. T cells that migrate across endothelial barriers and have accessed the meninges, CSF, and perivascular spaces will still need to bypass the glia limitans to reach the parenchyma. The glia limitans is the last barrier of the BBB and CXCL12 is important for retaining T cells within perivascular spaces and preventing their migration past the glia limitans. To breach this barrier, the scavenging of CXCL12 and the secretion of MMP2 and MMP9 by activated immune cells can permit T cells access to the parenchyma. However, in the context of brain tumors, disruption of the BBB may permit immune cell infiltration of the parenchyma without these steps. In EAE, evidence also suggests that DCs are critical for supporting both T cell infiltration and robust antigen-specific autoimmunity within the CNS. Preclinical models of brain tumor immunotherapy suggest that DCs recruited to the CNS may be instrumental for enhancing both T cell infiltration and retention within brain tumors.