Abstract
Due to advances in captive nonhuman primate (NHP) medical care, the number of geriatric chimpanzees (≥35 years old) is growing. With old age comes a variety of physical conditions, including arthritis, stroke, and mobility impairments. Programs aimed at enhancing the welfare of geriatric chimpanzees are now quite common, but there are few published empirical evaluations of the efficacy of such programs. The current study aimed to create, implement, and evaluate the effects of participation in a physical therapy (PT) program on physical health, mobility, welfare, and behavior. Nine chimpanzees with mobility impairments participated in personalized PT routines (using positive reinforcement training) twice per week for five months. Additionally, nine control chimpanzees (non-mobility impaired, matched with PT chimpanzees on age and sex) participated in body exam behavior sessions (also using positive reinforcement training) twice per week. All chimpanzees were rated on 14 health, well-being, and behavior items, as well as level of mobility throughout the PT program. Chimpanzees that participated in the PT program showed significant increases in ratings of physical health, well-being, and activity levels across phases of the program. Furthermore, compared to control chimpanzees, PT chimpanzees showed significant increases in ratings of ease of movement. Because raters were not blind to physical therapy treatment, our results represent an initial evaluation of the program that may suggest that participation in the PT program has physical, behavioral, and welfare benefits. Assessments of novel geriatric-focused care strategies and programs are essential to further enhance the welfare of the captive chimpanzee population, which is currently comprised of many geriatric animals, whose proportion of the captive population will only increase.
Keywords: Geriatric, Chimpanzee, Behavioral Management, Physical Therapy, Welfare
Introduction
Due to advancements in captive nonhuman primate (NHP) health care, the population of captive chimpanzees is increasingly experiencing advanced age, and geriatric (aged 35 years and older) chimpanzees are becoming more numerous (Dyke et al., 1995; Lowenstine et al., 2016; Nunamaker et al., 2012). These chimpanzees frequently face physical conditions related to old age, including arthritis, mobility impairments, stroke and resulting paresis, as well as injuries that require extended healing times (Kawanaka, 1993; Lacreuse et al., 2014; Lowenstine et al., 2016; Nunamaker et al., 2012). Captive chimpanzees also experience multiple behavioral changes during old age, including lower levels of affiliative and social behavior, less diversity in behavior, and higher levels of inactivity and anxiety-related behavior (Baker, 2000; Lacreuse et al., 2014; Neal Webb et al., 2019; Riopelle & Rogers, 1965; Tarou et al., 2002).
Behavioral and veterinary management programs are progressively focusing on techniques and strategies that aim to improve geriatric chimpanzee care. Personalized care routines that include acupuncture, laser therapy, and medication choice paradigms have resulted in chimpanzees voluntarily participating in their own health care, resulting in increased mobility and choice within the captive environment (Haller et al., 2012; Lambeth et al., 2013; Magden, 2017; Magden et al., 2013; Neal Webb et al., 2018). Evaluations of aspects of the physical and social environments of geriatric animals have led to structural enhancements to the physical environment and increased knowledge of the ways that social group composition affects geriatric chimpanzee behavior (Bridges et al., 2015; 2016; Neal Webb et al., 2019). More precise definitions of normal physiological parameters for aged individuals, including cholesterol, glucose, and lymphocyte counts, have led to enhanced veterinary understanding of typical aging in chimpanzees (Ely et al., 2013; Lowenstine et al., 2016; Nehete et al., 2017). Furthermore, the use of positive reinforcement training (PRT) to enhance the health care of older animals (by allowing chimpanzees to voluntarily participate in health care routines) has become a more common component of behavioral management and veterinary care procedures (Magden et al., 2013; 2016; Magden, 2017; Reamer et al., 2017; Prescott & Buchanan-Smith, 2003). For example, PRT has been used for voluntary presentation of body parts during acupuncture, laser therapy, blood glucose sampling, medication injection, and anesthetic injection for physical exams (Lambeth et al., 2006; Magden et al., 2013; 2016; Magden, 2017; Reamer et al., 2014).
Physical therapy may be an additional enhancement to captive geriatric chimpanzee care. In humans, physical therapy involves the treatment of disease, injury, or disability using physical methods (such as exercise), and has recognized benefits for a variety of conditions, including osteoarthritis, back pain, stroke, and injury, to name a few (Deyle et al., 2005; Gay et al., 2016; Geiger et al., 2001, Moseley, 2002; Skou et al., 2018). Therapies often include strengthening, range of motion, balance, and stretching exercises, up to three times per week (Jamtvedt et al., 2008; Vogel et al., 2009). Benefits are too numerous to outline here, but humans often experience increased walking speeds; improved balance; increased psychological well-being; reduced self-ratings of pain, dysfunction, and stiffness; and decreased levels of medication required to manage pain and discomfort as a function of PT (Deyle et al., 2005; Geiger et al., 2001, Jamtvedt et al., 2008; Moseley, 2002; Vogel et al., 2009). Overall, there is considerable evidence that strengthening exercises serve as an effective therapy to improve mobility and decrease pain, discomfort, and stiffness (Jamtvedt et al., 2008). Furthermore, the benefits of such physical exercises on the prevention of a variety of negative health outcomes (e.g., cancer, heart disease, diabetes, hypertension, stroke) are particularly pronounced in the elderly (Vogel et al., 2009). It is also worth noting that physical therapy can serve as an effective alternative to the use of drugs (e.g., opioids) for pain management (Mintken et al., 2018). These drugs often have addictive properties and side effects that are particularly pronounced in the elderly (including increased morbidity; Lee et al., 2018). Therefore, it is important to search for alternatives that do not simply treat symptoms, but also improve mobility and well-being.
Given that 1) older chimpanzees often experience physical conditions similar to those of older humans, and 2) physical therapy has wide-ranging benefits in humans, it follows that PT can be an effective refinement to captive chimpanzee care. Although programs aimed at enhancing the welfare of geriatric chimpanzees seem to be more common across captive settings (sanctuaries, zoos, and laboratories), there is limited published data assessing the efficacy of such programs. Therefore, the current study aimed to design, implement, and obtain an initial evaluation of a physical therapy program utilizing PRT to enhance the mobility and welfare of captive chimpanzees with documented mobility impairments.
Method
Subjects
Subjects were 18 captive chimpanzees living at the National Center for Chimpanzee Care (NCCC) at the Michale E. Keeling Center for Comparative Medicine and Research of The University of Texas MD Anderson Cancer Center in Bastrop, Texas. The Keeling Center has been continuously accredited by AAALAC International for over 40 years. Of the 18 chimpanzees (14 females, 4 males), nine were included in the physical therapy program (hereafter referred to as PT chimpanzees) and nine served as ‘control’ (see Procedure below for description) chimpanzees that were matched with PT chimpanzees on age (as closely as possible) and sex (Table 1). The nine PT chimpanzees ranged in age from 30 to 53 years (Mean Age = 45.77, SD = 7.44). One non-geriatric chimpanzee (ALL) was included as a PT chimpanzee due to her health condition (stroke and paresis).
Table 1.
Age, mobility impairment, initial exercise target, and matched control of each chimpanzee participating in the physical therapy program.
| PT Chimpanzee (Age/Sex) | Condition/Mobility Impairment | Initial Exercise Targets | Control Chimpanzee (Age/Sex) |
|---|---|---|---|
| *MAR (53/Female) | Arthritis: knees | 1 min 30 sec standing 10 squats |
TAN (50/Female) |
| *PEP (52/Female) | Arthritis: knees | 5 squats 1 min standing |
QUI (47/Female) |
| *SAB (51/Female) | Arthritis: knees/hips | 10 squats 1 min 30 sec standing |
ANG (44/Female) |
| *PRI (51/Female) | Stroke: right side paresis | 2 × 10 sec standing 2 climbs to upper bar 10 finger extensions |
SAN (47/Female) |
| *COR (46/Male) | Arthritis: hands, knees, hips, wrists, back |
1 min 30 sec standing 10 squats 10 weight shifts |
SIM (47/Male) |
| JOE (46/Male) | Previously healed injury to hand/finger causing limited movement and muscle tone |
20 weight shifts | AJA (40/Male) |
| *ROS (44/Female) | Arthritis: knees/hips | 10 squats 1 min 30 sec standing |
LUL (36/Female) |
| LAC (39/Female) | Stroke: slow movement, limited dexterity | 10 weight shifts | CAT (39/Female) |
| ALL (30/Female) | Stroke: left side paresis | 10 finger extensions 5 toe extensions |
CEC (28/Female) |
Indicates prescription of daily medication for pain management throughout all phases of the study.
Chimpanzees were housed in six separate, stable social groups, comprised of between 2 and 7 chimpanzees per group. Due to the NIH-mandated transfer of NCCC chimpanzees to Chimp Haven, the national chimpanzee sanctuary, which required the disruption of some social groups, two of the PT chimpanzees were temporarily housed in pairs. Several PT and control chimpanzees were housed together as follows: Group A: ALL, LAC, and ROS; Group B: PEP, PRI, and QUI; Group C: MAR and CEC; Group D: AJA and JOE; Group E: LUL and SAN; and Group F: TAN and CAT. Subjects ANG, COR, SAB, and SIM also lived in social groups, however their groups did not include any other study animals.
Chimpanzees were housed in Primadomes™ or corrals with indoor/outdoor access. Both Primadomes™ and corrals included various physical environmental enrichment items, including, but not limited to, wooden climbing structures with platforms, telephone poles, culvert sections, tractor tires, various sizes of plastic balls, 55-gallon barrels, and fire hose rope/swings (Neal Webb et al., 2018a). Chimpanzees were also provided with daily foraging opportunities, enrichment devices, positive human interaction, and positive reinforcement training. The research conducted in this study complied with the approved protocols of the UTMDACC Institutional Animal Care and Use Committee, and adhered to the legal requirements of the United States and to the American Society of Primatologists’ Principles for the Ethical Treatment of Primates.
Procedure
Two trainers and one behavioral researcher served as the therapists for the physical therapy program (hereafter referred to as the therapy team). The therapy team identified the nine chimpanzees for the physical therapy program on the basis of the presence of mobility impairments due to physical conditions or past physical history (e.g., stroke, arthritis, previous injury; Table 1). Therefore, PT chimpanzees exhibited mobility impairments, moderate to high levels of slow movement, moderate to severe crepitation, veterinarian-diagnosed arthritis and prescription of anti-inflammatory drugs, and/or limited movement due to previous injury or stroke. All chimpanzees with mobility impairments were included as subjects in the PT program in order to maximize sample size and so as not to withhold a potentially beneficial refinement from mobility-impaired animals (Neal Webb et al., 2018b). Control chimpanzees also experienced slight mobility issues due to their age (even the single 30-year old subject: CEC), including slight crepitation (as reported by veterinarians during physical exams within the past two years) and some slow movement. However, they did not exhibit a level of impairment that warranted prescription of medication or classification as mobility-impaired by a veterinarian.
With the approval of the veterinarians and colony manager, the therapy team created initial exercises for each chimpanzee based on 1) the specific mobility impairment, 2) past physical history or injury, 3) intended benefits to the chimpanzee (e.g., increased agility, strength), and 4) feasibility to complete the exercises each week (e.g., therapist schedules, ability of the chimpanzees to perform exercises). These exercises (see Table 1) consisted of behaviors that could be trained using PRT techniques. All chimpanzees were previously trained on multiple behaviors, including sit, stand, climb, present hand, and present foot (Schapiro, Perlman, Thiele, & Lambeth, 2005), and the therapists used and built on these behaviors to create exercises. For example, several chimpanzees had an initial exercise target of 10 squats. For this exercise, the therapist would give the “stand” cue to the chimpanzee, reward the chimpanzee with a half of a grape for standing, then give the cue to “sit”. Upon sitting, the therapist would reward the chimpanzee with another half of a grape. This process was repeated 10 times. Another common exercise was “weight shifting”. Therapists would ask the chimpanzee to climb up the mesh so that the chimpanzee was at eye level with the therapist. Then, the therapist alternated the left and right “present hand” cues, which required that the chimpanzee shift its weight back and forth between the left and right arm/hand while hanging from the opposite hand (see supplemental material for video clips of example exercises).
The following is one example of the process we used to determine the initial exercises for one chimpanzee. Chimpanzee PEP exhibited slow and stiff lower limb movement due to arthritis. The therapy team aimed to increase strength and flexibility in the lower limbs through squats and standing exercises. During the Baseline assessment, it was noted that PEP began “sinking” after standing for 1 minute and 15 seconds, and exhibited increased respiratory rates after 7 squats. Therefore, the therapy team began PEP’s initial targets at 1 minute of standing and 5 squats to avoid possible over-exertion and potential injury.
The therapy team also recorded a Baseline video of each chimpanzee performing its initial exercises. These exercises were implemented within therapy sessions for five weeks (Phase 1, see Table 2). At the end of Phase 1, the therapy team assessed each chimpanzee’s progress and exercises, and recorded a follow-up video of the completion of his or her respective exercises. Based on the chimpanzee’s progress, the therapy team either increased or decreased the number of repetitions for each exercise, changed the type of exercises within the regimen, or left the regimen as it was. This process was repeated for Phases 2–5 (each lasting five weeks). Each therapist was responsible for the physical therapy regimen of three PT chimpanzees, as well as PRT sessions with three control chimpanzees. PT chimpanzee therapy sessions were approximately 4–5 minutes in length.
Table 2.
Dates of study phases and assessments.
| Study Phase (Activity) | Phase Date Range (2019) | Relevant Assessment Date (2019) |
|---|---|---|
| Baseline Assessment | March 19 | |
| Phase 1 | April 1 - May 11 | May 7 |
| Phase 2 | May 12 – 15 | June 13 |
| Phase 3 | June 16 – July 20 | July 16 |
| Phase 4 | July 21 – August 17 | August 9 |
| Phase 5 | August 17 – September 21 | September 20 |
| Phase 6 through ongoing Phases | September 22 – present | Ongoing |
Note: During each phase, PT sessions occurred twice per week. Assessments consisted of progress assessments and appropriate adjustments of exercises.
After the start of the PT program, we wanted to attempt to isolate the effects of the physical therapy exercises from any effects due to additional attention and/or PRT. Therefore, at phase 3, we chose the 9 control animals (matched as closely on age as possible) and began control training sessions. These training sessions were approximately the same length as therapy sessions with PT chimpanzees, and consisted of various PRT body exam behaviors twice per week.
Participation in therapy and control training sessions was voluntary and took place while the animals were living in their social group. If a chimpanzee chose not to participate in a particular therapy session, the therapist revisited the chimpanzee later in the day to request participation again. If the ape still chose not to participate, the therapist returned the following day or later in the week. Throughout the PT program, there were only five cases across all PT chimpanzees in which non-participation resulted in a single therapy session during a given week.
Caregiver wellness ratings
Throughout each phase (described above), as well as one month prior to the start of PT (Baseline), each chimpanzees’ assigned caregiver was asked to complete a wellness rating sheet for that animal, consisting of 14 subjective items (see Table 3). The wellness items utilized a 1–5 scale, with 1 representing “Never” or “Unacceptable”, and 5 representing “Always” or “Excellent” for frequency and subjective items, respectively. Therefore, caregivers provided a single rating for each of the 14 items on the wellness rating sheet for their assigned animals (see below). These items were chosen because they address our specific interest in measuring the effects of PT on behavior and wellness. During phases 1 – 3, caregivers completed one rating sheet (i.e., one sheet with 14 items) for their subject chimpanzees once per week. During phases 4 and 5, caregivers completed one rating sheet per chimpanzee each month due to difficulty in ensuring that caregivers completed the ratings each week. Wellness ratings for control chimpanzees began during phase 3 of the PT program, since PRT with, and observations of, these animals did not begin until phase 3 (see above).
Table 3.
Wellness rating sheet items and example behaviors.
| Item | Examples |
|---|---|
| Overall physical health | Subjective rating of medical condition, disease, injury fitness status, etc. |
| Well-being | Subjective rating of comfortable, happy, positive, etc. |
| Activity level | Subjective rating of overall amount of physical activity |
| Ease of movement | Subjective rating of stiffness, slow movement, etc. |
| Abnormal | Frequency of pacing, rocking, head twirl, hair plucking, etc. |
| Anxiety-related | Frequency of scratching, jumpy, vigilant, etc. |
| Depressive | Frequency of hunched posture, lack of reaction to groupmates and humans, etc. |
| Affiliative | Frequency of embrace, hand-to-body contact, pant-grunts, etc. |
| Grooming (give) | Frequency of giving grooming |
| Grooming (receive) | Frequency of receiving grooming |
| Play | Frequency of chase, rough and tumble, play face, poke, etc. |
| Interaction with enrichment | Frequency of enrichment use |
| Fear-related | Frequency of fear grimace, scream, avoid/flee, etc. |
| Aggressive | Frequency of display, charge, hit, slap, etc. |
The behavioral researcher (part of the therapy team) trained caregivers on rating procedures and items prior to the start of the program. Training included examples of behaviors and ratings for each item, as well as instructions regarding the importance of objectivity and consistency in ratings. Caregivers were instructed to complete rating sheets based on 1) their understanding of that specific chimpanzee, 2) their understanding of general chimpanzee behavior, and 3) their observations and impressions of that chimpanzee’s behavior and well-being over a specified time period; during the past week (phases 1–3) or past month (phases 4–6). Each caregiver was assigned specific PT and control chimpanzees (based on their assigned care area) for which they completed wellness rating sheets. For example caregiver AM completed wellness rating sheets for chimpanzees PEP, PRI, QUI, LAC, ALL, ROS, and SAB throughout the duration of the study. Caregivers were very familiar with the behavior and history of each of their assigned chimpanzees, as caregivers had worked with these chimpanzees for a minimum of two years, and familiarity with the behavior and well-being of their animals is part of the job description at the NCCC. During the PT program, although caregivers may have briefly seen a PT session in progress as they were completing other daily duties, they did not explicitly observe PT sessions and were not told days and times of PT sessions. Additionally, therapists attempted to limit their discussion of PT routines and chimpanzee progress with caregiver raters throughout the study to avoid influencing caregiver ratings.
Mobility scores
Three individuals (colony manager, trainer, and veterinary research assistant) rated each PT chimpanzee’s overall mobility using the mobility scoring system (Neal Webb et al., 2018b) once per month. Due to both a lack of personnel and work-related time constraints, mobility scoring did not begin until Phase 3 of the PT program. Briefly, the mobility scoring system is comprised of six categories of mobility rated on a scale of 1–5, in which numerically higher scores are indicative of greater impairments in mobility. The mean of the three raters was used in analyses. Inter-rater reliability was acceptable (Intraclass correlation = 0.82; Fleiss & Cohen, 1973). Control chimpanzees were not rated on this system, as no control chimpanzees exhibited mobility impairments, and as such, would have received the lowest score possible, indicating the least impairment in mobility (Neal Webb et al., 2018b).
Annual physical exams
Due to NIH regulations regarding which types of data can be collected for research purposes, we opportunistically examined unprompted clinical notes from veterinarians during annual physical exams. Therefore, we determined the date of each chimpanzee’s last annual physical exam to occur prior to the start of the PT program (before 1 April 2019), and called this their pre-PT physical exam. We then determined the date of their first physical exam to occur at least two months following the start of the PT program (1 June 2019), and called this their post-PT annual physical exam. We then looked in the database (i.e., veterinarian notes) for clinical notes that pertained to arthritis, crepitation, inflammation, previous injury, and muscle measurements. We present qualitative data for these variables.
Data analysis
We averaged wellness ratings within each phase to create a mean for each of the 14 items for Baseline and Phases 1, 2, 3, 4, and 5 (or Baseline and Phases 3, 4, and 5 for control chimpanzees). We first aimed to confirm that control and PT chimpanzees differed in wellness ratings at Baseline. Therefore, within the Baseline ratings, we used a series of bootstrapped independent samples t–tests to examine differences in ratings of physical health, overall well-being, activity levels, ease of movement, and depressive, play, and anxiety-related behaviors.
Within PT chimpanzees, we used repeated-measures ANCOVAs (age as covariate) to examine differences in overall physical health, well-being, ease of movement, activity, mobility scores, as well as depressive, play, and anxiety-related behaviors, over time (Baseline through Phase 5).
Lastly, given that wellness ratings for control chimpanzees began during Phase 3 of the PT program, analyses comparing wellness ratings between control and PT chimpanzees were limited to Baseline and Phases 3, 4, and 5. Therefore, we used mixed-model ANCOVAs with control/PT chimpanzee as the between-subjects factor, Phase (Baseline, 3, 4, 5) as the within-subjects factor, and age as covariate in all models to examine differences in wellness items and mobility as a function of Phase and participation in the PT program. Follow-up pairwise comparisons were used for post-hoc tests or to assess a priori hypotheses. To correct for multiple comparisons, but to avoid overcorrection resulting in Type II Error (Nakagawa, 2004; Perneger, 1998), p-values ≤ 0.01 were considered significant, and 0.02 ≤ p ≤ 0.07 were considered trending. All analyses were performed using SPSS Statistics 24 (IBM Corporation, Chicago, IL, USA). Means and standard errors are reported. Where appropriate, corrected values for t, F, df, and p for unequal variances are reported. Data are available from the corresponding author upon reasonable request. The data are not publicly available due to privacy restrictions.
Results
Wellness ratings
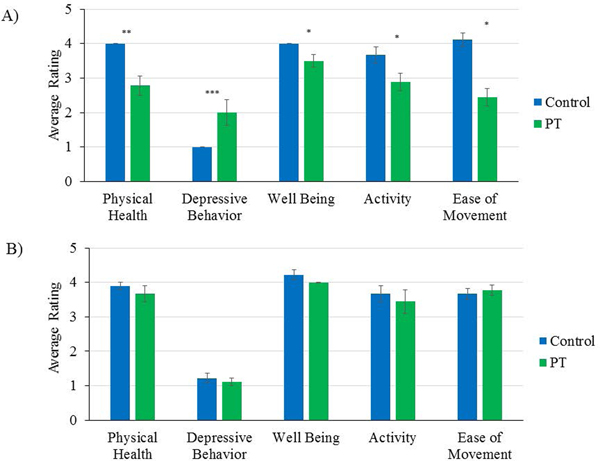
Bootstrapped independent samples t-tests showed several differences between control and PT chimpanzees at Baseline. Compared to control chimpanzees, PT chimpanzees showed significantly lower ratings of physical health [t(8)=4.40, p = 0.002] and ease of movement [t(16)=5.30, p = 0.0001], and a trend toward lower ratings of activity levels [t(16)=2.21, p = 0.04], well-being [t(8)=2.53, p = 0.03], and higher depressive behavior [t(8)=−2.68, p = 0.028] (see Figure 1A). These differences in behavioral ratings between PT and control chimpanzees were no longer significant by the end of the phases included in this study (Phase 5: p > 0.10; Figure 1B).
Figure 1.
A) Differences in wellness ratings between control and PT chimpanzees at Baseline. Error bars represent standard error of the mean. * p < 0.05; ** p < 0.01; *** p < 0.001. B) Differences in wellness ratings between control and PT chimpanzees at Phase 5. Error bars represent standard error of the mean. Note that none of the comparisons are significant (p > 0.10)
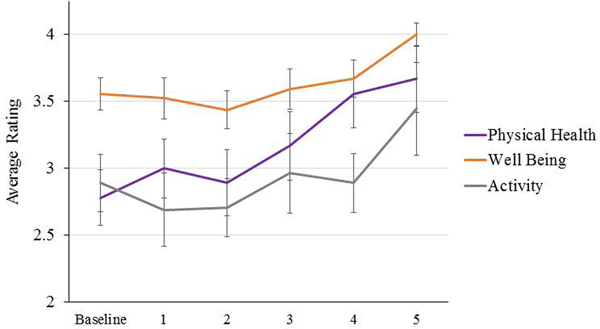
Within PT chimpanzees, repeated measures ANCOVAs showed significant increases over the course of the PT program in average ratings of physical health [F(5,35)=3.96, p = 0.006], activity levels [F(5,35)=3.68, p = 0.009], and well-being [F(5,35)=5.13, p = 0.001] (Figure 2). Pairwise comparisons showed that chimpanzee physical health ratings were higher during Phase 5 (M = 3.67, SE = 0.25) compared to Baseline (M = 2.78, SE = 0.21, p = 0.03) and Phase 2 (M = 2.89, SE = 0.25; p = 0.01), and were also higher during Phase 4 (M = 3.56, SE = 0.26) compared to Baseline (p = 0.01), Phase 1 (M = 3.00, SE = 0.22; p = 0.018), and Phase 2 (p = 0.003). Chimpanzee activity levels were highest during Phase 5 (M = 3.44, SE = 0.35), although only the comparison with Phase 2 (M = 2.70, SE = 0.22) was trending toward significance (p = 0.02). Lastly, ratings of well-being were higher during Phase 5 (M = 4.00, SE = 0.01) compared to all other phases, including Baseline (M = 3.56, SE = 0.12, p = 0.008), Phase 1 (M = 3.52, SE = 0.15, p = 0.016), Phase 2 (M = 3.44, SE = 0.14, p = 0.006), Phase 3 (M = 3.59, SE = 0.15, p = 0.03), and Phase 4 (M = 3.67, SE = 0.14, p = 0.047).
Figure 2.
Differences in ratings of PT chimpanzee physical health, well-being, and activity across phases of the PT program. Error bars represent standard error of the mean.
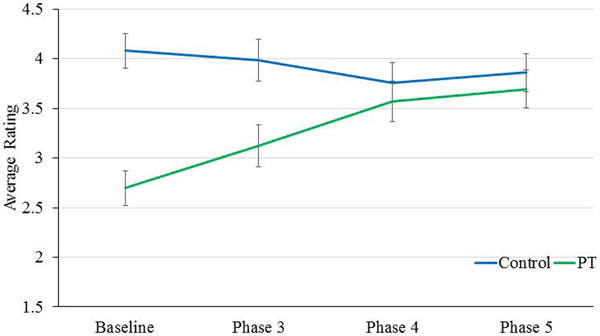
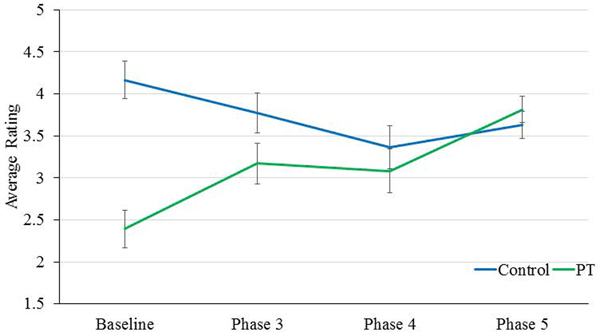
Finally, between control and PT chimpanzees across Baseline and Phases 3, 4, and 5, a mixed-model ANCOVA showed a significant interaction between Phase and control/PT chimpanzee for physical health [F(3,45)=6.30, p = 0.001]. While control chimpanzees’ ratings of physical health remained approximately the same across phases, PT chimpanzees’ ratings showed steady increases across phases (Figure 3). There was also a significant Phase by control/PT chimpanzee interaction for ease of movement [F(3,45)=9.15, p = 0.001], whereby PT chimpanzees’ ratings showed increases across phases, while control chimpanzees showed a slight decrease across phases (Figure 4). There were no other significant or trending main or interaction effects.
Figure 3.
Differences between control and PT chimpanzee physical health ratings across phase of the PT program.
Figure 4.
Differences between control and PT chimpanzee ease of movement ratings across phase of the PT program.
Mobility scores
There was a trend toward lower mobility scores during Phase 4 (M = 9.85, SE = 1.06) (remember, lower mobility scores are indicative of fewer impairments in mobility) compared to scores during Phase 5 (M = 9.04, SE = 0.96, p = 0.035). Phase 3 mobility scores (M = 8.94, SE = 1.04) were not significantly different from Phase 4 or Phase 5 of the program (p > 0.10).
Annual physical exams
Of the nine PT chimpanzees, seven met pre- and post-PT physical exam criteria, and of these, three chimpanzees had clinical notes in both pre- and post-PT exams that could be used as qualitative data for the current study. During chimpanzee PEP’s pre-PT exam (13 June 2018), it was noted that PEP exhibited crepitation in both stifle joints; during the post-PT exam (mid-Phase 5: 5 September 2019), crepitation was reported in only the left stifle joint. Compared to chimpanzee PRI’s pre-PT exam (8 March 2018), muscle measurements during her post-PT exam (end of Phase 2: 11 June 2019) showed 4 cm and 2 cm increases in measurements of the right thigh and calf, respectively (the side of paresis), as well as a 2 cm increase of the left thigh and 2 cm decrease of the left calf. Lastly, during chimpanzee MAR’s pre-PT exam (26 July 2018), “severe” crepitation was noted in both stifles, whereas only “moderate” crepitation was noted in both stifles during the post-PT exam (mid-Phase 2: 5 June 2019).
Discussion
The aim of the current study was to design, implement, and carry out an initial evaluation of a physical therapy program for mobility-impaired chimpanzees. Unfortunately, we could not implement a blind-rating system due to the nature of the physical therapy program (i.e., we were unable to “hide” physical therapy sessions from caregivers). Therefore, we present and interpret our findings as an initial assessment of the PT program, as we have plans to implement blind ratings in the future. Regardless, chimpanzees that participated in personalized physical therapy routines were rated as showing increases in physical health, activity levels, and well-being across the five-month PT program. Additionally, compared to control chimpanzees, PT chimpanzees were rated as showing significant increases in ratings of ease of movement and physical health. These results suggest that chimpanzees participating in the PT program are experiencing several benefits, including enhancements in activity levels, physical health, and movement abilities, as well as an overall increase in welfare. Importantly, this seems to be the result of the therapy regimen itself, as control chimpanzees that participated in training sessions of approximately equal length per week showed no such increases in these ratings.
At the National Center for Chimpanzee Care, we have implemented several personalized care routines aimed at refining captive care (Bridges et al., 2013; 2015; 2016; Haller et al., 2012; Lambeth et al., 2013; Magden et al., 2013; 2016; Reamer et al., 2017; Neal Webb et al., 2018). This physical therapy program, using PRT, represents an additional important refinement that can be applied to a variety of NHP species experiencing a variety of physical conditions, including arthritis, paresis, lameness, and injury. In addition to enhancing physical health, physical therapy routines may be enriching to NHPs in other ways, as the procedures increase the level of human interaction and choice within the captive environment, both of which have been shown to enhance welfare (Baker, 2004; Pomerantz & Terkel, 2009; Reamer et al., 2014). In the current study, control chimpanzees received a comparable amount of increased human interaction and choice through training sessions, but did not show the same increases in wellness ratings as PT chimpanzees (although control chimpanzees did show a trending decrease in anxiety-related behavior ratings across phases, perhaps suggesting that there were some beneficial effects of the training sessions alone). Therefore, it seems as though the increased human interaction and choice did not account for the results found in the current study (although increases in human interaction and choice may represent an additional benefit of the physical therapy program).
At Baseline, PT chimpanzees were rated as showing several differences in wellness ratings compared to control chimpanzees, including lower ratings of physical health, well-being, activity, and ease of movement. Additionally, they were rated as having higher levels of depressive behavior. It is unknown how these behaviors may influence one another; for example, how depressive behavior may influence activity levels, whether lower ease of movement may cause depressive behavior, etc. Regardless of the direction of these relationships, these findings suggest that the PT chimpanzees (i.e., older chimpanzees with past medical conditions and mobility issues) were likely experiencing lower levels of well-being than their less-impaired counterparts, particularly prior to the start of the PT program. The difference between PT and control chimpanzees in these ratings was almost non-existent by Phase 5 of the study (Figure 1B), suggesting that the level of welfare of PT chimpanzees was comparable to the level of welfare of control chimpanzees after participation in the PT program. Unexpectedly, the control group was rated as showing a decrease in ease of movement across the study. Notes left on the wellness rating sheets suggest that this decrease is likely due to agonistic interactions and injuries within groups containing control subjects, particularly in three groups (housing four control animals and no PT animals) in which female reproductive swellings likely increased levels of aggression within the group.
Overall, the PT program is relatively easy to implement, although flexibility is a necessary attribute for the success of the program. Each chimpanzee’s session was approximately 4–5 minutes in length. With two sessions per week, the time commitment for each therapist was approximately 30 minutes each week. This amount of time was occasionally extended due to a chimpanzee’s non-participation on any particular day, demonstrating the importance of remaining flexible in therapy routines and schedules. However, therapists reported that, the majority of the time, chimpanzees were eager to participate. Additionally, flexibility and adaptability may be needed to accommodate the needs of veterinarians (e.g., physical exams, treatments), behavioral managers (e.g., (re)introductions), caregivers, and therapists (e.g., changes in scheduling, care staff shortages, etc.).
It is imperative that methods and/or data collection protocols be in place to ensure that there are no negative consequences of PT, including injury, decreased mobility, or increased aggressive behavior within the social group as a result of increased attention toward a particular animal. In the current program, the therapy team met throughout, and at the end of, each phase, to ensure that chimpanzees were not being pushed too hard or too quickly (e.g., signs of difficulty breathing, shakiness, wincing, inability to complete PT session, etc.), and to address any potential physical or behavioral consequences of the PT routine on the animals. The program also utilized a team approach, including requesting 1) veterinarian and colony manager approval, 2) caregivers’ complete wellness ratings, and 3) a behavioral researcher be trained on PRT techniques in order to complete therapy sessions and collect empirical data. This not only dispersed program duties across the team, but also ensured that there were multiple people involved in monitoring each chimpanzee’s well-being throughout the program.
We chose to include all chimpanzees with mobility issues in the PT program in order to maximize sample size, and, more importantly, avoid withholding a potentially beneficial treatment from mobility-impaired animals (Neal Webb et al., 2018b). The finding that control chimpanzees showed no major changes in ratings of ease of movement and physical health suggest that the exercises themselves seem to be affecting these ratings in PT chimpanzees (with the caveat that we were unable to use blind raters). Additionally, the finding that PT animals had ratings similar to those of control chimpanzees by Phase 5, further suggests that the PT is beneficial, perhaps helping to reduce the differences in well-being between mobility-impaired chimpanzees and their non-mobility-impaired counterparts.
Given that weight management for captive primates can be challenging, particularly for those who are older and less active, there may be some concern regarding additional food rewards distributed throughout the PT program. In the current program, some food rewards were supplemental and some were part of the daily allotment. Juices used to complete standing exercises were part of daily “hydration”. Grapes were supplemental, but just one grape was used per repetition per exercise, amounting to approximately 60 extra grapes per animal per week. For chimpanzees that were overweight, grapes were often cut in half. Additionally, chimpanzees maintained high motivation with just one grape per exercise repetition, and as such, we have not had to increase the reward value of the foods used. However, given that we did not weigh chimpanzees before, during, or after the PT program, we cannot explicitly state that they did not gain weight during the program.
One limitation of the current study was that caregivers were not blind to the identities of the chimpanzees that were receiving physical therapy. However, therapists limited discussion with caregivers regarding the PT program, methodology, exercises, chimpanzee progress, and, particularly, any results. The full extent of the caregivers’ involvement in the project was that they were simply asked to complete the wellness rating sheets. Regardless of the lack of information that the caregiver raters received regarding PT and the chimpanzees, the potential for bias exists. Since this is an ongoing project, we plan to alter the rating protocol to include blind raters in upcoming phases of the program. As such, the results presented here should be considered an initial evaluation of this PT program.
An additional limitation was that our assessment of physiological effects of the physical therapy program was limited to opportunistic qualitative data obtained from a limited number of annual physical exams. Furthermore, some of the pre-PT physical exams occurred up to one year prior to the start of the PT program, and therefore did not coincide with the timing of the program. Although there were no major changes in social groupings or physical health between pre- and post-PT exams, we are unable to rule out other circumstances that may have influenced the clinical notes used as data in this study. Regardless, it is worth noting that the three chimpanzees for which there were qualitative data, were three of the four oldest PT chimpanzees. Unfortunately, we are unable to infer whether this suggests that older chimpanzees experience greater benefits of the program due to the opportunistic nature of these data. Therefore, experimental measures of physiological-type effects of the physical therapy program (e.g., muscle tone, crepitation, inflammation) are needed.
The physical therapy program at the NCCC is ongoing, and we expect further improvements in mobility, health, and behavior. We plan to implement increased frequency and duration of physical therapy sessions in future phases of the program. Given that ratings showed some benefits with just 8–10 minutes of physical therapy per week, it is possible that increasing this amount of time (e.g., 20 minutes per week), while remaining cognizant of the animals’ physical limitations, will have additional benefits to both well-being and mobility. Like physical therapy for humans, physical therapy for chimpanzees is a cost-effective health care strategy that improves health outcomes and may also help to prevent certain conditions that are common with old age (e.g., hypertension, stroke, diabetes; Bürge et al., 2016; Vogel et al., 2009). Importantly, assessments of programs aimed at geriatric care should become more commonplace to determine whether the techniques and programs are indeed having their intended effects and to further refine the care of an aging captive chimpanzee population.
Supplementary Material
Acknowledgements.
This research was supported by NIH U42-OD-011197. Thank you to Drs. Elizabeth Magden and Stephanie Buchl for input on, and approval of, the physical therapy program. Thank you to Amanda Felchak, Shannon Andrews, Katie Smaus, and Alicia Foley for completing wellness rating sheets and to Laurie Mason and Rachel Haller for completing Mobility Scores. Thank you to all NCCC staff for unwavering support of and care for the chimpanzees. All work was carried out in accordance with the care and use of animal guidelines as laid out by the American Society of Primatologists, and was approved by the Institutional Animal Care and Use Committee at UTMDACC. We have no conflicts of interest to declare.
References
- Baker KC (2000). Advanced age influences chimpanzee behavior in small social groups. Zoo Biology, 19(2), 111–119. [DOI] [Google Scholar]
- Baker KC (2004). Benefits of positive human interaction for socially-housed chimpanzees. Animal Welfare, 13(2), 239. [PMC free article] [PubMed] [Google Scholar]
- Bridges JP, Mocarski EC, Reamer LA, Lambeth SP, & Schapiro SJ (2013). Weight management in captive chimpanzees (Pan troglodytes) using a modified feeding device. American Journal of Primatology, 75(S1), 51. [Google Scholar]
- Bridges JP, Haller RL, Buchl SJ, Magden ER, Lambeth SP, & Schapiro SJ (2015). Establishing a behavioral management program for geriatric chimpanzees. American Journal of Primatology, 77(S1), 111–111. [Google Scholar]
- Bridges JP, Powers KM, Lambeth SP, & Schapiro SJ (2016). Assessment of structure use and structural modifications for geriatric chimpanzees (Pan troglodytes). Presented at the Joint meeting of the International Primatological Society and the American Society of Primatologists: August 21–27, 2016, Chicago, IL. [Google Scholar]
- Bürge E, Monnin D, Berchtold A, & Allet L. (2016). Cost-effectiveness of physical therapy only and of usual care for various health conditions: Systematic review. Physical Therapy, 96(6), 774–786. 10.2522/ptj.20140333 [DOI] [PubMed] [Google Scholar]
- Deyle GD, Allison SC, Matekel RL, Ryder MG, Stang JM, Gohdes DD, ... & Garber MB. (2005). Physical therapy treatment effectiveness for osteoarthritis of the knee: a randomized comparison of supervised clinical exercise and manual therapy procedures versus a home exercise program. Physical therapy, 85(12), 1301–1317. 10.1093/ptj/85.12.1301 [DOI] [PubMed] [Google Scholar]
- Dyke B, Gage TB, Alford PL, Swenson B, & Williams‐Blangero S. (1995). Model life table for captive chimpanzees. American Journal of Primatology, 37(1), 25–37. 10.1002/ajp.1350370104 [DOI] [PubMed] [Google Scholar]
- Ely JJ, Zavaskis T, & Lammey ML (2013). Hypertension increases with aging and obesity in chimpanzees (Pan troglodytes). Zoo Biology, 32(1), 79–87. 10.1002/zoo.21044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleiss JL, & Cohen J. (1973). The equivalence of weighted kappa and the intraclass correlation coefficient as measures of reliability. Educational and Psychological Measurement, 33(3), 613–619. 10.1177/001316447303300309 [DOI] [Google Scholar]
- Gay C, Chabaud A, Guilley E, & Coudeyre E. (2016). Educating patients about the benefits of physical activity and exercise for their hip and knee osteoarthritis. Systematic literature review. Annals of Physical and Rehabilitation Medicine, 59(3), 174–183. 10.1016/j.rehab.2016.02.005 [DOI] [PubMed] [Google Scholar]
- Geiger RA, Allen JB, O’Keefe J, & Hicks RR (2001). Balance and mobility following stroke: Effects of physical therapy interventions with and without biofeedback/forceplate training. Physical Therapy, 81(4), 995–1005. 10.1093/ptj/81.4.995 [DOI] [PubMed] [Google Scholar]
- Haller RL, Magden ER, Lambeth SP, & Schapiro SJ (2012). Using positive reinforcement techniques to train for acupuncture treatment in chimpanzees (Pan troglodytes): An innovative approach in managing an aging colony. American Journal of Primatology, 74(S1), 43. [Google Scholar]
- Jamtvedt G, Dahm KT, Christie A, Moe RH, Haavardsholm E, Holm I, & Hagen KB (2008). Physical therapy interventions for patients with osteoarthritis of the knee: An overview of systematic reviews. Physical Therapy, 88(1), 123–136. 10.2522/ptj.20070043 [DOI] [PubMed] [Google Scholar]
- Lacreuse A, Russell JL, Hopkins WD, & Herndon JG (2014). Cognitive and motor aging in female chimpanzees. Neurobiology of Aging, 35(3), 623–632. 10.1016/j.neurobiolaging.2013.08.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambeth SP, Hau J, Perlman JE, Martino M, & Schapiro SJ (2006). Positive reinforcement training affects hematologic and serum chemistry values in captive chimpanzees (Pan troglodytes). American Journal of Primatology 68, 245–256. 10.1002/ajp.20148 [DOI] [PubMed] [Google Scholar]
- Lambeth SP, Schapiro SJ, Bernacky BJ, & Wilkerson GK (2013). Establishing ‘quality of life’ parameters using behavioural guidelines for humane euthanasia of captive non-human primates. Animal Welfare, 22(4), 429–435. 10.7120/09627286.22.4.429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JC, Miller MD, Karp J, & Reynolds CF (2018). Strategies for reducing the overuse of prescription drugs in elders: A focus on opiods and benzodiazepines. The American Journal of Geriatric Psychiatry, 26(3), S12 10.1016/j.jagp.2018.01.021 [DOI] [Google Scholar]
- Lowenstine LJ, McManamon R, & Terio KA (2016). Comparative pathology of aging great apes: Bonobos, chimpanzees, gorillas, and orangutans. Veterinary Pathology, 53(2), 250–276. 10.1177/0300985815612154 [DOI] [PubMed] [Google Scholar]
- Magden ER (2017). Positive reinforcement training and health care In Schapiro SJ (Ed.), Handbook of Primate Behavioral Management (pp. 201–215). CRC Press, Taylor & Francis Group: Boca Raton, FL, USA: 10.1201/9781315120652-13 [DOI] [Google Scholar]
- Magden ER, Haller RL, Thiele EJ, Buchl SJ, Lambeth SP, & Schapiro SJ (2013). Acupuncture as an adjunct therapy for osteoarthritis in chimpanzees (Pan troglodytes). Journal of the American Association for Laboratory Animal Science, 52, 475–480. [PMC free article] [PubMed] [Google Scholar]
- Magden ER, Sleeper MM, Buchl SJ, Jones RA, Thiele EJ, & Wilkerson GK (2016). Use of an implantable loop recorder in a chimpanzee (Pan troglodytes) to monitor cardiac arrhythmias and assess the effects of acupuncture and laser therapy. Comparative Medicine, 66, 52–58. [PMC free article] [PubMed] [Google Scholar]
- Mintken PE, Moore JR, & Flynn TW (2018). Physical therapists’ role in solving the opioid epidemic. Journal of Orthopaedic & Sports Physical Therapy, 48(5), 349–353. 10.2519/jospt.2018.0606 [DOI] [PubMed] [Google Scholar]
- Moseley L. (2002). Combined physiotherapy and education is efficacious for chronic low back pain. Australian Journal of Physiotherapy, 48(4), 297–302. 10.1016/s0004-9514(14)60169-0 [DOI] [PubMed] [Google Scholar]
- Nakagawa S. (2004). A farewell to Bonferroni: The problems of low statistical power and publication bias. Behavioral Ecology, 15(6), 1044–1045. 10.1093/beheco/arh107 [DOI] [Google Scholar]
- Neal Webb SJ, Hau J, & Schapiro SJ (2018a). Captive chimpanzee (Pan troglodytes) behavior as a function of space per animal and enclosure type. American Journal of Primatology, 80(3), e22749. 10.1002/ajp.22749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neal Webb SJ, Hau J, & Schapiro SJ (2018b). Refinements to captive chimpanzee (Pan troglodytes) care: A self-medication paradigm. Animal Welfare, 27(4), 327–341. 10.7120/09627286.27.4.327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neal Webb SJ, Hau J, Lambeth SP, & Schapiro SJ (2019). Differences in behavior between elderly and non-elderly captive chimpanzees and the effects of the social environment. Journal of the American Association of Laboratory Animal Science, 58(6), 783–789. 10.30802/aalas-jaalas-19-000019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nehete PN, Magden ER, Nehete BP, Williams LE, Abee CR, & Sastry KJ (2017). Age-and sex-associated differences in phenotypic and functional characteristics of peripheral blood lymphocytes in chimpanzees (Pan troglodytes). Journal of the American Association for Laboratory Animal Science, 56(5), 509–519. [PMC free article] [PubMed] [Google Scholar]
- Nunamaker EA, Lee DR, & Lammey ML (2012). Chronic diseases in captive geriatric female chimpanzees (Pan troglodytes). Comparative medicine, 62(2), 131–136. [PMC free article] [PubMed] [Google Scholar]
- Perneger TV (1998). What’s wrong with Bonferroni adjustments. BMJ: British Medical Journal, 316(7139), 1236 10.1136/bmj.316.7139.1236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomerantz O, & Terkel J. (2009). Effects of positive reinforcement training techniques on the psychological welfare of zoo-housed chimpanzees (Pan troglodytes). American Journal of Primatology, 71, 687–695. 10.1002/ajp.20703 [DOI] [PubMed] [Google Scholar]
- Prescott MJ, & Buchanan-Smith HM (2016). Training nonhuman primates using positive reinforcement techniques. Journal of Applied Animal Welfare Science, 6(3), 157–161. 10.1207/s15327604jaws0603_01 [DOI] [PubMed] [Google Scholar]
- Reamer L, Haller R, Thiele EJ, Freeman HD, Lambeth SP, & Schapiro SJ (2014). Factors affecting initial training success of blood glucose testing in captive chimpanzees (Pan troglodytes): Chimpanzee diabetic treatment training. Zoo Biology, 33, 212–220. 10.1002/zoo.21123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reamer L, Haller R, Lambeth SP, & Schapiro SJ (2017). Behavioral management of Pan spp In Schapiro SJ (Ed.), Handbook of Primate Behavioral Management (pp. 395–407). CRC Press, Taylor & Francis Group: Boca Raton, FL, USA: 10.1201/9781315120652-23 [DOI] [Google Scholar]
- Riopelle AJ, & Rogers CM (1965). Age changes in chimpanzees. In: A.M. Schrier, H.F. Harlow, and F. Stollnitz (Eds.), Behavior of Nonhuman Primates: Modern Research Trends (pp. 449–462). Academic Press: New York, New York, USA. 10.1016/b978-1-4832-2821-1.50012-4 [DOI] [Google Scholar]
- Schapiro SJ, Perlman JE, Thiele E, & Lambeth S. (2005). Training nonhuman primates to perform behaviors useful in biomedical research. Lab Animal, 34(5), 37–42. 10.1038/laban0505-37 [DOI] [PubMed] [Google Scholar]
- Skou ST, Pedersen BK, Abbott JH, Patterson B, & Barton C. (2018). Physical activity and exercise therapy benefit more than just symptoms and impairments in people with hip and knee osteoarthritis. Journal of Orthopaedic & Sports Physical Therapy, 48(6), 439–447. 10.2519/jospt.2018.7877 [DOI] [PubMed] [Google Scholar]
- Tarou LR, Bloomsmith MA, Hoff MP, Erwin JM, & Maple TL (2002). The behavior of aged great apes In Erwin JM & Hoff PR (Eds.), Interdisciplinary Topics in Gerontology: Aging in Nonhuman Primates, Vol 31 (pp. 209–231). Karger Publishers: Basel, Switzerland. 10.1159/000061467 [DOI] [Google Scholar]
- Vogel T, Brechat PH, Leprêtre PM, Kaltenbach G, Berthel M, & Lonsdorfer J. (2009). Health benefits of physical activity in older patients: A review. International Journal of Clinical Practice, 63(2), 303–320. 10.1111/j.1742-1241.2008.01957.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.