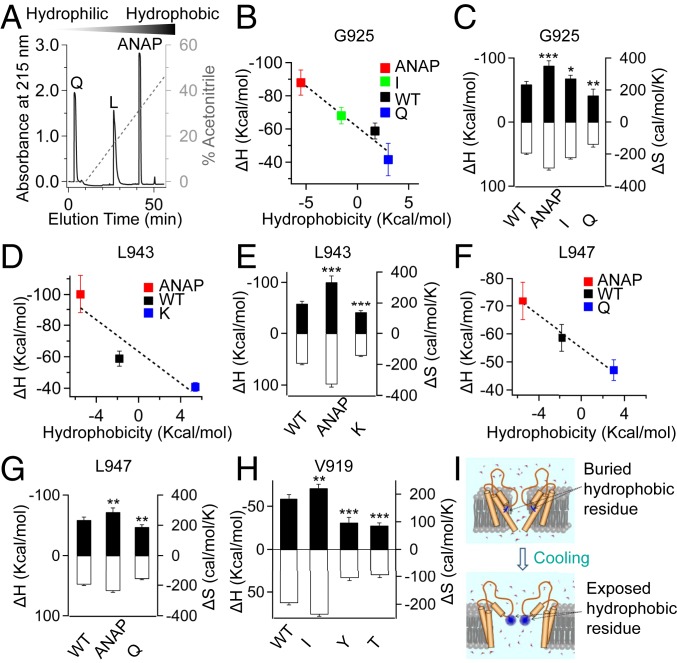
Fig. 4.
SCH of residues located in the PD tunes the cold sensitivity of TRPM8. (A) Relative hydrophobicity of amino acids was compared by a C18 reverse-phase high-performance liquid chromatography column. (B–G) SCH of residues located in sites 925, 943, and 947 of TRPM8_LA positively correlates with the measured ΔH values. Measured ΔH values (filled bars, left axis) and ΔS values (open bars, right axis) of wild-type TRPM8_LA and channel mutants (mean ± SEM; *P < 0.05, **P < 0.01, ***P < 0.001; n = 5). (H) Measured ΔH (filled bars, left axis) and ΔS values (open bars, right axis) of wild-type TRPM8_LA and its mutants with point substitution at site 919 (mean ± SEM; **P < 0.01, ***P < 0.001; n = 5). (I) A schematic illustration where a hydrophobic residue transits from the buried (Top) to exposed state (Bottom).