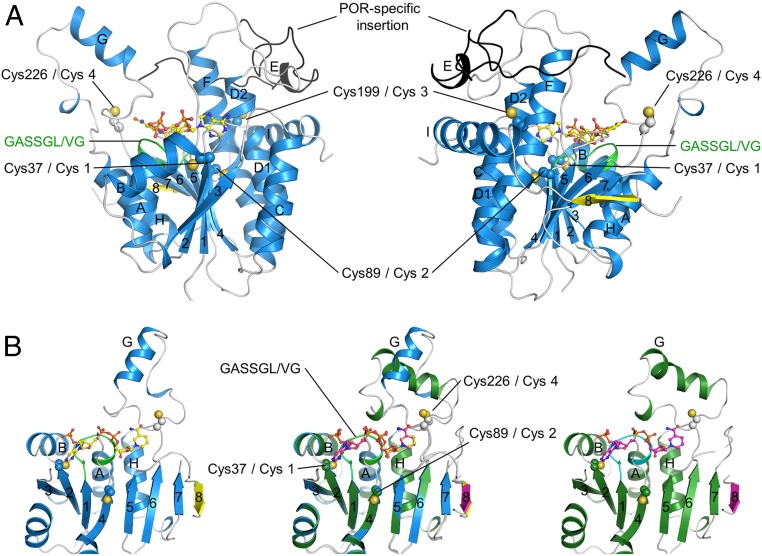
Fig. 1.
Ribbon representation of the overall structures of SyLPOR and TeLPOR. (A) Two side views of SyLPOR. The secondary structure elements are colored in blue except the antiparallel β8 in yellow. The loop region is in gray. The LPOR-specific insertion is colored in black. The NADPH-binding sequence is colored in green. Four cysteine residues are shown in sphere mode. The cofactor NADPH is shown in stick-and-ball mode. (B) Front view of SyLPOR (Left), TeLPOR (Right), and their superimposition (Middle). The secondary structure elements of TeLPOR are colored in deep green except β8 in magenta; the NADPH-binding sequence is colored in cyan. The α-helices are labeled alphabetically, and the β-strands are labeled numerically.