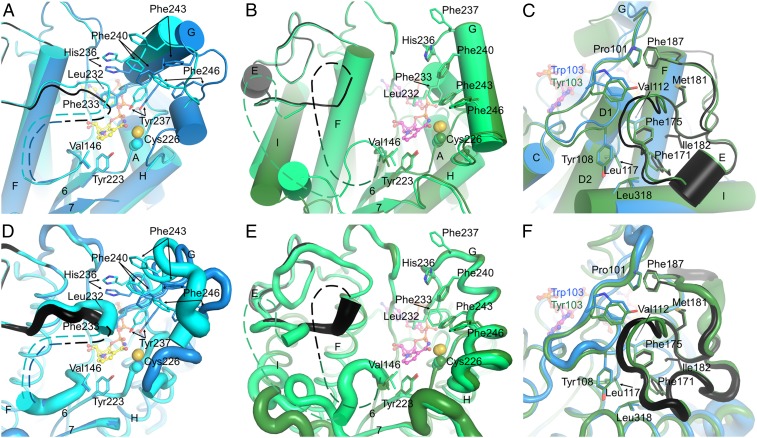
Fig. 3.
The substrate-binding site and the LPOR-specific insertion. (A) The substrate cavity of SyLPOR. The two polypeptide chains in an asymmetric unit are superimposed and colored blue and cyan. For clarity, only one NADPH molecule is shown (yellow stick). The side chains of residues possibly participating in Pchlide binding are shown as sticks. The LPOR-specific insertion of one chain is colored black. The missing fragment is depicted as dashed lines. (B) The substrate cavity of TeLPOR. The polypeptide chains are colored deep green and green, and only one NADPH is shown (magenta). Other representations are same as in A. (C) The majority of the LPOR-specific insertion is folded with the αβα-core. The core-insertion interacting residues are shown as sticks. SyLPOR and TeLPOR, respectively, are colored blue and deep green except that the insertion sequence in SyLPOR is colored black. (D–F) Representation of the flexibility of A, B, and C. The backbone is shown as tubes whose radius corresponds to the temperature factor of the Cα-atoms.