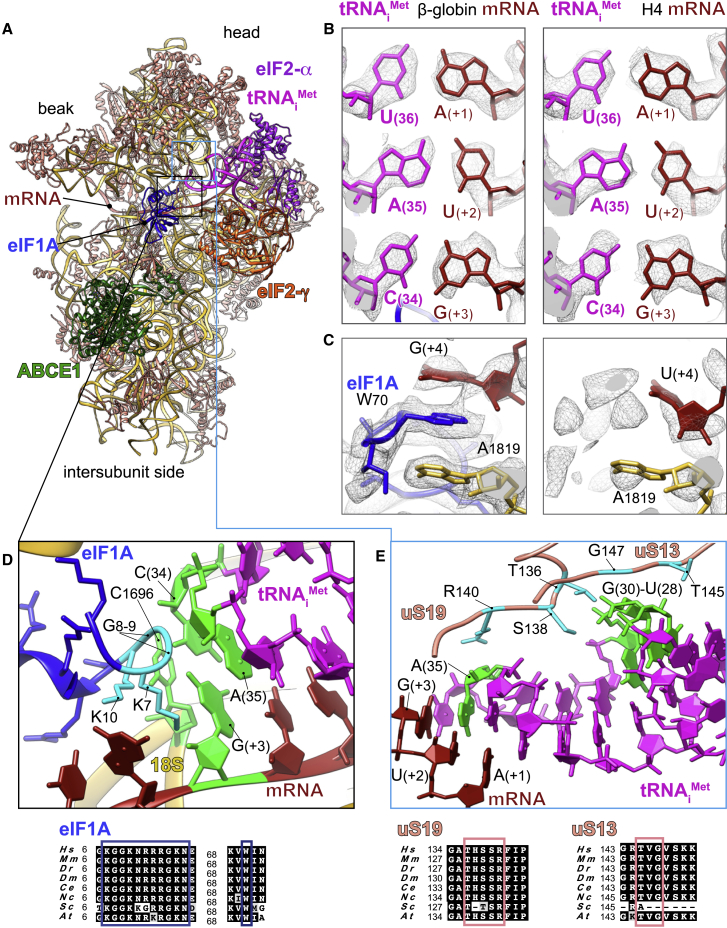
Figure 2.
Key Interactions Surrounding the Start-Codon Recognition Sites in β-Globin and H4 LS48S ICs
(A) Ribbon representation of the atomic model of β-globin LS48S IC viewed from the intersubunit side.
(B) Codon:anticodon base-pairing view in both mRNA complexes; left: β-globin; right: H4.
(C) eIF1A (sky blue) interaction with the mRNA in the β-globin IC (left panel), compared with the corresponding region in the H4 IC, which is mostly free of eIF1A (right panel).
(D) Close-up of the eIF1A N-terminal tail (colored in cyan) showing its intricate interactions with tRNA and mRNA; stacking of C1696 on tip of tRNAiMet. The nucleotides involved in the interactions are colored green.
(E) Interaction network of the tRNAi with ribosomal proteins uS13 and uS19 (colored salmon). Residues involved in the interactions are colored cyan in uS13 and uS19 and green in the tRNAi. For eIF1A, uS13, and uS19, sequence alignments of the concerned interacting regions from eight representative eukaryotic species are shown below the panels in black boxes and the described residues are indicated by colored frames.
Hs, Homo sapiens; Mm, Mus musculus; Dr, Danio rerio; Dm, Drosophila melanogaster; Ce, Caenorhabditis elegans; Nc, Neurospora crassa; Sc, Saccharomyces cerevisiae; At, Arabidopsis thaliana.