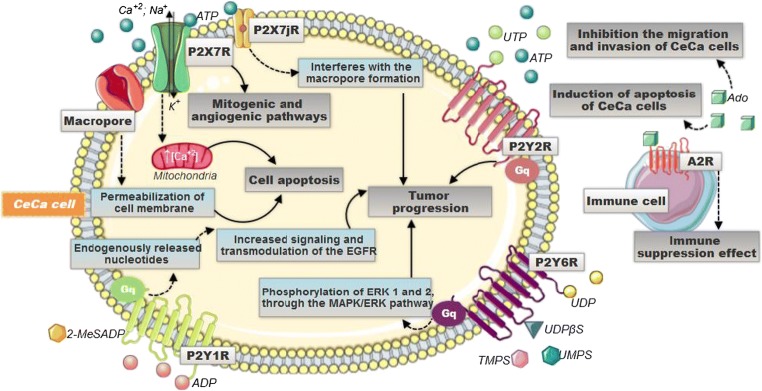
Fig. 3.
Different mechanisms are triggered when stimulated by the different nucleotides in the CeCa TME. P2X7R, when activated, promotes an increase in intracellular ATP production and, via PI3K-AKT, through the signaling dependent on HIF1α, triggers mitogenic and angiogenic pathways, stimulating tumor progression. P2Y-type receptors are coupled to protein G and activate the PLC pathway. When the P2Y2R is activated, it leads to an increase in cell proliferation, while the P2Y6R, through the MAPK/EK1,2 pathway, also participates in processes that promote cellular proliferation. On the other hand, P2Y1R mediates the mitogenic signals of endogenously released nucleotides through transactivating the epidermal growth factor receptor (EGFR) pathway—which is related to cell differentiation and proliferation and, ultimately, also promotes tumor progression. Adenosine, present in high amounts in the TME, leads to the activation of A2a receptor in T lymphocyte cells, which promotes the decrease of proliferation, activation, and effector function of these cells