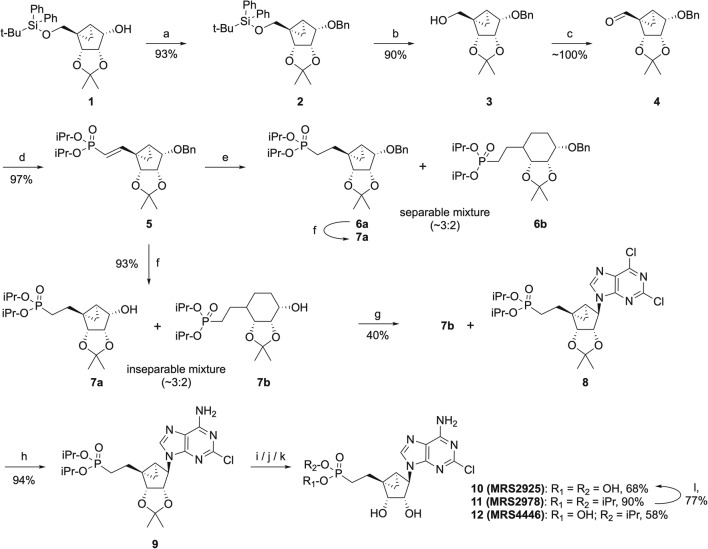
Fig. 2.
Synthesis of MRS2925 and MRS2978. Reagents and conditions: (a) DMF, NaH, BnBr; 0 °C → rt., 2 h; (b) THF, TBAF, rt., 3 h; (c) CH2Cl2, Dess-Martin reagent, rt., 3 h; (d) THF, tetra-isopropyl methylene diphosphonate, BuLi, − 40 °C → − 20 °C → rt., 1.5 h; (e) methanol, TEA, H2-Pd/C, rt., 18 h; (f) methanol, H2-Pd/C, rt., 18 h; (g) THF, 2,6-dichloropurine, PPh3, DIAD, rt., 48 h; (h) i-PrOH-NH3, 70 °C, 18 h; (i) CH2Cl2, TMSI, rt., 18 h; (j) MeOH-H2O (1:1), Dowex-50 H+-Form, 55 °C, 3 h; (k) CH2Cl2, TMSI, rt., 45 min; (l) 3 mM aq. HCl, μwave, 130 °C, 60 min