Abstract
For centuries, the Acetobacteraceae is known as a family that harbors many species of organisms of biotechnological importance for industry. Nonetheless, since 1988 representatives of this family have also been described as nitrogen fixing bacteria able to plant growth promotion by a variety of mechanisms. Nitrogen fixation is a biological process that guarantees that the atmospheric N2 is incorporated into organic matter by several bacterial groups. Most representatives of this group, also known as diazotrophic, are generally associated with soil rhizosphere of many plants and also establishing a more specific association living inside roots, leaves, and others plants tissues as endophyte. Their roles as plant growth‐promoting microorganisms are generally related to increase in plant biomass, phosphate and other mineral solubilization, and plant pathogen control. Here, we report many of these plant growth‐promoting processes related to nitrogen fixing species already described in Acetobacteraceae family, especially Gluconacetobacter diazotrophicus and their importance to agriculture. In addition, a brief review of the state of art of the phylogenetics, main physiological and biochemical characteristics, molecular and functional genomic data of this group of Acetobacteraceae is presented.
Keywords: Biological nitrogen fixation, Plant growth promotion, Gluconacetobacter diazotrophicus, Acetic acid bacteria
Introduction
Bacteria belonging to the Alphaproteobacteria, order Rhodospirillales, are known for their agricultural applicability. This order is represented by two bacterial families: Rhodospirillaceae and Acetobacteraceae. In general, the etymology of members of the Acetobacteraceae family derives from the Latin acetum or acidum gluconicum + bacter due to their peculiar main characteristic to produce organic acids during many biotechnological processes, such as vinegar and wine productions. Bacteria of the genus Acetobacter are known since 1898 with the description of A. aceti 1, 2. However, only at 1988, it means 100 years after the first species description in this family, is that a diazotrophic species was described in this family 3. At that time, this discovery raised the possibility that bacteria of many other species could also present nitrogen fixing and plant growth promotion properties for agricultural purpose, similar to the rhizobia inoculant for soybeans. This document brings the trajectory of accumulated knowledge about the species Gluconacetobacter diazotrophicus and other diazotrophs genera and species that were described later in this family.
The Acetobacteraceae family
Bacteria belonging to the Acetobacteraceae family (ex Henrici, 1939) 4 are classified as rods (coccus or ellipsoidal), Gram‐negative, mobile, aerobic that conduct an incomplete oxidation of sugars and alcohols to produce organic acids as final product of their metabolism. They have the ability to grow in very acidic environments with pH close to 3.0–3.5, but the optimum range is 5.0–6.5 5. They are commonly known as “Acetic Acid Bacteria” (AAB) due to their use in the production of vinegar, but this is not the only biotechnological product derived from these bacteria as reviewed in Raspor and Goranovic 6. Among them, we can mention the production of several fermented beverages, for example, beverages derived from coconut cream, cocoa products of fermentation, in addition to producing microbial cellulose, biotransformation of glucose to gluconic acid and its ketoderivatives, production of non‐caloric sugar d‐tagalose and shikimate that is an intermediate in the production of many antibiotics. Nonetheless, the AAB may act in the opposite way in a few drinks and pathogenic to diverse cultures being the causative agent of diseases in pineapple and apple. Its presence can influence the quality of beverages such as beer, wine, and vinegar, since certain amount of oxygen and optimum temperature can promote further oxidation of ethanol and organic acids. On the other hand, representatives of the genera Acetobacter, Roseomonas, and Granulibacter have been considered opportunistic bacteria related to chronic granulomatosis, pneumonia, and bacteremia, respectively 7, 8, 9, 10, 11, 12, 13.
Acetobacteraceae family initially corresponded to the representatives of the genera Acetobacter and Gluconobacter, based on morphological, physiological, and biochemical criteria 14, 15. In 1950, Frateur 16 proposed a classification based on the following physiologic criteria: catalase production, gluconic acid production from glucose, acetic acid oxidation to CO2 and H2O, lactic acid oxidation to CO2 and H2O, and the oxidation of glycerol to dihydroxyacetone. Later on, hybridization studies of DNA:rRNA indicated that Acetobacter and Gluconobacter formed a single and independent branch of the RNA superfamily justifying their unification in Acetobacteraceae family 4. In 1984, Yamada and Kondo 17 proposed a new subgenus denominated Gluconoacetobacter, which was elevated to the category of genus based on the analysis of 16S rRNA gene sequences 18. In accordance to this reclassification, species such as Acetobacter diazotrophicus, Acetobacter europaeus, Acetobacter hansenii, Acetobacter liquefaciens, and Acetobacter xylinus were transferred to the new genus Gluconoacetobacter, which then was corrected to Gluconacetobacter 19. The genus Acetobacter shows quite unique and exceptional predominance of quinone type Q‐9, while quinone type Q‐10 is predominant in the other genera 5. Many of these bacteria are capable of performing a wide variety of biotechnological processes, but those most important commercially are related to the genera Acetobacter, Gluconacetobacter, and Gluconobacter.
To date, the genera described and recognized at the LPSN‐list of prokaryotic names with standing in nomenclature are Acetobacter, Acidicaldus, Acidiphilium, Acidisoma, Acidisphaera, Acidocella, Acidomonas, Ameyamaea, Asaia, Belnapia, Craurococcus, Endobacter, Gluconacetobacter, Gluconobacter, Granulibacter, Humitalea, Komagataeibacter, Kozakia, Muricoccus, Neoasaia, Neokomagataea, Paracraurococcus, Rhodopila, Rhodovarius, Roseococcus, Roseomonas, Rubritepida, Saccharibacter, Stella, Swaminathania, Tanticharoenia, Teichococcus, and Zavarzinia 20. In addition, Nguyenibacter 21, Rhodovastum 22, Sediminicoccus 23, and Swingsia 24 are other proposed genera that remain to be formally recognized and are in the list of prokaryotic names without standing in nomenclature.
Peculiarities of some Acetobacteraceae genera
Some peculiarities that deviate from the classical description of AAB can be highlighted on the genera and their species described over these last 50 years.
The genus Rhodopila was proposed by Imhoff et al. 25 to accommodate a group of purple non‐sulfur bacteria that presents vesicular intracytoplasmic membranes, similar to Rhodobacter species, but grows at low pH. The genus Acidiphilium was described after isolation and characterization of heterotrophic, mesophilic bacteria with requirements for high acidity and unable to use elemental sulfur or ferrous 26. Later on, isolates from acidic hot springs and mine drainage were characterized on the basis of molecular and phenotypic traits, especially related to ion‐chelated chlorophyll a type, in the genus Acidisphaera, which differs from other aerobic bacteriochlorophyll‐containing (ABC) bacteria by producing zinc‐chelated bacteriochlorophyll a (Zn‐BChl) 26, 27, 28. In addition to them, other genera known to present bacteriochlorophyll a type were also phylogenetically clustered into the family Acetobacteraceae, and they are as follows: Roseomonas 7, 29, Roseococcus 30, Craurococcus, Paracraurococcus 31, Rubritepida 32, Humitalea 33, and Rhodovastum (proposed but not yet recognized genus) 22.
The genus Acidocella was proposed to accommodate two previously described Acidiphilum species that do not present BChl a and that have been classified as monophyletic unit apart from other Acidiphilium species according to 16S rDNA sequence 34, 35.
The genus Stella is known as a polyprosthecate bacteria characterized by having numerous appendage (from the Greek prostheca) 36. Although it has being classified in this family, further studies based on polar lipids and 16S rRNA genes of the two type strains species indicated its close relationship to members of Rhodospirillaceae 37.
The characterization of a group of acidophilic methanol‐utilizing bacteria leads to reclassification of Acetobacter methanolicus into a new genus, Acidomonas 38. Afterwards, an emendation of the genus description stated that Acidomonas presents acid tolerance, instead of acidophily 39.
Representatives of the genus Asaia do not grow in presence of methanol, are characterized by poor or non‐existent production of acetic acid from ethanol, and by the absence of growth in presence of 0.35% acetic acid (w/v) 40. The genera Asaia, Kozakia, Swaminathania, and Neoasaia are related phylogenetically to each other; however, Tsp509I and MboII restriction of the 16S–23S rDNA ITS can differentiate Asaia species from Kozakia and Neosaia‐type strains 41.
The genera Muricoccus and Theichococcus were proposed to accommodate isolates that do not produce bacteriochlorophyll a under aerobic conditions and were obtained from building material of a children's day care center, specifically from gypsum liner walls of a children's sleeping room 42. However, Sanchez‐Porro et al. had suggest that T. ludipueritiae and M. roseus, the single species of each genera, should be placed within the genus Roseomonas based on pigment color, carbon metabolism, and fatty acids profiles 29.
The genus Saccharibacter corresponds to isolates able to grow in the presence of high concentrations of glucose (2–40% w/v—optimum at 10% w/v) but that present negligible or very weak productivity of acetic acid from ethanol. The species type strain S. floricola was isolated from pollen collected in Kanagawa, Japan 43.
The genus Acidisoma comprises two species, A. tundrae and A. sibiricum, that were isolated from acidic Sphagnum‐dominated tundra and Siberian wetlands in Russia 44. As general characteristic, they are chemoorganotrophic strict aerobes, psychrotolerant, do not possess bacteriochlorophyll a, produce poly‐β‐hydroxy‐butyrate, and are oxidase‐ and catalase‐positive.
Representative of the genus Nguyenibacter, N. vanlangensis, was isolated from rhizosphere of rice plants in Vietnam 21. The main characteristics of this genus are oxidation of acetate to carbon dioxide and water but not lactate, no production of acetic acid from ethanol; growth is weakly positive either on 30% d‐glucose (w/v) or in the presence of 0.35% acetic acid (w/v). As isolation procedure, the authors used LGI N‐free‐medium prior to cultivation onto another medium containing glucose and ethanol as carbon source and pH 3.5 but nitrogen fixation was not assessed.
Two genera were proposed during classification of isolates obtained from a flower sample from Thailand, the genus Neokomagatea that comprises N. thailandica and N. tanensis 45 and the genus Swingsia that comprises the species S. samuiensis, but is not formally recognized 24. Endobacter medicaginis, the first Acetobacteraceae found as legume nodules endophytes, was isolated from surface sterilized nodules of Medicago sativa grown in an acidic soil at the Province of Zamora, Spain 46.
Several phenotypic and genotypic studies served as the basis for reclassification of several species previously described in the Gluconacetobacter genus at the generic level, including nitrogen fixing species 46, 47, 48, 49, 50. The genus Komagataeibacter were proposed during separation of Gluconacetobacter xylinum group from the Gluconacetobacter liquefaciens group.
To date, among all Acetobacteraceae genera only some representatives of the genera Gluconacetobacter, Acetobacter, Komagataeibacter, Swaminathania, Asaia, and Acetobacter are reported as nitrogen fixing bacteria and the strategies used in order to obtain these new species are described in Table 1 3, 47, 51, 52, 53, 54, 55, 59.
Table 1.
Media utilized for isolation of nitrogen fixing Acetobacteraceae
| Enrichment medium | |||||||
|---|---|---|---|---|---|---|---|
| Genus | Bacterial species | N‐free LGIa | SPYCb | EM Ic | EM IVd | GYPe | Initial pH |
| Gluconacetobacter | G. diazotrophicus | + cane juice | − | − | − | − | 4.5 |
| G. johannae | + | − | − | − | − | 5.7 | |
| G. azotocaptans | + | − | − | − | − | 5.7 | |
| Asaia | A. siamensis | − | + | − | − | − | 3.5 |
| A. bogorensis | − | + | − | − | − | 3.5 | |
| A. platycodi | − | − | + | + | − | 3.5 | |
| Swaminathania | S. salitorerans | + NaCl | − | − | − | − | 5.5 |
| Komagataeibacter | K. (kombuchae) hansenii | + antifungif | − | − | − | − | 4.5 |
| K. kakiaceti | − | − | + | – | + antifungi | 3.5 | |
| Acetobacter | A. nitrogenifigens | + antifungi | − | − | − | − | 4.5 |
| A. peroxydans | + Yg | − | − | − | − | 6.0 | |
LGI semi‐solid medium containing 10% raw sugar from sugarcane 3.
SPYC—2.0% d‐sorbitol,0.5% peptone, 0.3% yeast extract, 0,0001% cycloheximide.
EM I—1.0% glucose, 0.5% ethanol, 1.5% peptone, 0.8% yeast extract, 0.3% acetic acid, 0.1% cycloheximide, pH 3.5.
EM IV—2.0% ducitol, 0.5% peptone, 0.3% yeast extract, 0.1% cycloheximide, pH3.5.
GYP—1.0% glucose, 1% glicerol, 1% peptone, 0.5%, 0,7% CaCO3.
Antifugi—cycloheximide and/or nystatin (150 mg L−1 each).
The Gluconacetobacter genus
Until late 80s, there was not any report of AAB capable to fix nitrogen but Cavalcante and Döbereiner 3 isolated on N‐free semi‐solid media and described the first N2‐fixing AAB. They succeed to isolate from sugarcane plants a group of acid‐tolerant bacteria able to fix nitrogen even at pH below 3.5 using a minimal medium based on LG medium 64, named LGI‐P medium, that presents 10% of raw sugar as carbon source and pH around 5.5.
Afterwards, during phylogenetic study of representatives of the genus Acetobacter, a new genus was proposed by Yamada et al. 18 leading to the elevation of the subgenus Gluconoacetobacter to the generic level. Two species previously classified as Acetobacter were then renamed into this new genus, G. liquefaciens and G. xylinus, based on 16S RNA sequence, predominance of Q‐10 quinone type, flagella, pigment production, cellulose production, and fatty acid profile. Later on, during the validation, the name has been corrected to Gluconacetobacter in accordance with Rule 61 of the Bacteriological Code 19. Since then, a plethora of species isolated from various environments were described into this genus. Nonetheless, after detailed phylogenetic analysis some of them were reclassified into another genus 47, 51, 55, 65, 66, 67, 68, 69.
After 2001, new nitrogen fixing species were described in the genus Gluconacetobacter, G. johannae, and G. azotocaptans 51. G. swingsii and G. rhaeticus are cellulose‐producing acetic acid bacteria isolated from apple juice of fruits cropped in the South Tyrol region of Italy 67. G. sacchari was isolated from sugarcane and insects 66. G. saccharivorans and G. nataicola were proposed based on a reclassification study of Gluconacetobacter hansenii strains 70. G. tumulicola and G. asukensis were isolated from biofilms growing on the surface of the plaster walls of the mural paintings of the Kitora Tumulus in Japan 68. G. tumulisoli, G. takamatsuzukensis, and G. aggeris were isolated from the burial mound soil collected at Takamatsuzuka Tumulus in Asuka village, Nara Prefecture, Japan 69. As previously occurred to G. kombuchae, the species G. kakiaceti 62, G. medellinensis 71, and G. maltaceti 72 were reclassified into the genus Komagataeibacter 55 based on previous phylogenetic studies using 16S rRNA sequences 47, 50.
The reasons that corroborated the existence of two phylogenetic groups in the genus Gluconacetobacter was discussed by Yamada and Yukphan 13. According to previous observations, the group 1 included G. liquefaciens, G. diazotrophicus, G. sacchari, G. johannae, and G. azotocaptans, while group 2 included G. xylinus G. hansenii, G. europaeus, G. entanii, G. oboediens, G. intermedius, G swingisii, G rhaeticus, G. saccharivorans, and G. nataicola. Group 1 species differentiate from group 2 by many physiological and morphological traits, such as flagella and motility, water‐soluble brown pigment production, production of γ‐pyrone compounds, and 2,5 di‐ketogluconic. Species clustered into these groups also showed other common features such as biotechnological applications and habitats. Ecologically, the species of group 1 were found associated with plants, fruits, or flowers, noteworthy group 2 were generally isolated from fermentation process and processed food, such as vinegar, Kombucha tea, and nata de coco 73. Nitrogen fixation agents of biocontrol and associated to several plant growth promotion were related to species of group 1, while most species in group 2 were related with industrial applications. Cleenwerck et al. 74 also contributed with genotypic data to reinforce the separation of Gluconacetobacter species at generic level. Recently, G. xylinum group (group 2) were separated from the G. liquefaciens group (group 1) leading to reclassification of several species in the genus Komagataeibacter, including G. kombuchae, a nitrogen fixing species later considered heterotypic synonym of previously named G. hansenii 48, 50.
In the present scenario, the ability to fix nitrogen has being observed in the following Gluconacetobacter species: G. diazotropicus 3, 75, G. johannae, and G. azotocaptans 51.
Gluconacetobacter diazotrophicus
The species Gluconacetobacter diazotrophicus (former Acetobacter diazotrophicus) was isolated from roots, stems, and leaves of sugarcane not only in Brazil but also in Argentina, Uruguay, Mexico, Cuba, United States, India, Canada, Egypt, beside others 3, 76, 77, 78 and then from other agricultural crops such as sugar beet, rice, pineapple, coffee, carrot, and many others 79, 80, 81. It was also isolated from bugs, such as mealybugs commonly found associated to sugarcane crops 82, 83.
This species has been considered as one of the most important diazotrophic bacteria found in high numbers (104–106 CFU g−1 plant fresh tissues) and colonizing the inner parts of roots, stems, and leaves of sugarcane 84. It is a nitrogen‐fixing bacterium originally classified as Acetobacter diazotrophicus but later renamed to the genus Gluconacetobacter based on the 16S rDNA sequence and the predominant type of ubiquinone 18, 19.
Physiological characteristics
It is Gram‐negative, aerobic but fixes nitrogen in microaerobic conditions. It does not use tricarboxylic acids to grow and is adapted to conditions of high osmolarity and sucrose content (10–30%). In addition, its cultivation in presence of high sugar content revealed that nitrogenase activity is only partially inhibited by the addition of ammonium to the culture medium 85. It shows high acidity tolerance, fixes nitrogen in presence of nitrate concentrations greater than 10 mM, and reduces the deleterious effect of oxygen concentration to the nitrogenase activity using oxidative metabolism in the periplasmic space at membrane level 3, 86.
Genetic traits
Studies of genetic characterization in G. diazotrophicus started in the early 90s. The first report of the chromosomal localization of nitrogen fixation genes and presence of plasmids in G. diazotrophicus strains was presented during the 6th International Nitrogen fixation with non‐legumes in Egypt 87. Later on, the nif genes, transcription regulatory genes (nifA and ntrBC) and others related to ammonium sensing and transport (glnB, glnD, and amtB) were sequenced and expression of some of them were studied using transcriptional gusA fusion 88, 89, 90, 91, 92. The nif‐fix gene organization found in G. diazotrophicus was similar to that of Azospirillum brasilense, but their products were homologous to those observed to representatives of Rhizobiaceae family and R. capsulatus, considering the GenBank data available at that time 88, 91.
In addition, accessory genes related with nitrogen sensing and metabolism were also studied by sequencing and insertion mutagenesis. At least three copies of genes coding PII‐like protein were identified. The copy located upstream to glnA (that codes for glutamine synthetase) was named glnB, because of its homology and conserved organization on many others nitrogen‐fixing bacteria. The others PII‐like coding genes, glnK1 and glnK2, were located upstream of copies of genes that codes for ammonium or methylammonium transportes, amtB1 and amtB2, respectively 92. Further characterization of single and double mutants containing gus‐fusion suggested that GlnB and GlnK1 are required as positive signals to efficiently relieve repression by GlnK2. In addition, the authors showed that GlnK2 protein clearly has a function different from those of GlnB and GlnK1, since nif gene expression were repressed in glnB glnK1 double mutant under all conditions tested. Based on these studies, the authors suggested that none of the three PII homologs is required for nif gene expression indicating novel regulatory features of G. diazotrophicus PII proteins. The GlnK2 protein acts primarily as an inhibitor of nif gene expression while GlnB and GlnK1 control the expression of nif genes in response to ammonium availability, both directly and by relieving the inhibition by GlnK2 92.
Some years ago a lab consortium, named RIOGENE, announced that the genome of this microorganism was sequenced 60. Its genome is composed of a 3.9 Mb chromosome and 2 plasmids of 16.6 and 38.8 kb, respectively. The genome size is in the average of others AAB genome already published 93, 94, 95, 96, 97, 98, 99. Further studies of this microorganism functional genomics have been underway by several researchers group and are leading to a comprehension of the role of its gene contents to its general metabolism, nitrogen fixation, and others plant growth mechanisms 100, 101, 102, 103, 104, 105, 106, 107, 108. In addition, several mutants have already been obtained and used for functional genomic characterization 100, 102, 103, 104, 105, 106, 107, 109.
The expression of genes related to reactive oxygen species (ROS) detoxification (sod, kat, and gor) was evaluated in G. diazotrophicus grown under nitrogen non‐fixing (NFIX) and fixing conditions to elucidate the paradox of oxygen consumption during respiration protection and ROS inhibitory effect on nitrogenase activity and nif gene expression 110. They observed that growth of this microorganism under nitrogen fixing condition leads to reduction of ROS accumulation and a strong induction of sodA, katE, kat, katC, and gorA genes in comparison to cells grown under non‐fixing condition. In addition, sodA and katE gene expression correlates with nifD suggesting that reduction of ROS is essential for the homeostasis during respiratory protection and nitrogen fixation in G. diazotrophicus. Later on, the participation of G. diazotrophicus detoxifying genes during the first step of root colonization was investigated using rice plants 111. It was shown that ROS accumulates during the first steps of inoculation of G. diazotrophicus wild type and mutant strains as response of a plant defense mechanism. Noteworthy, they found out that GR (glutathione reductase) and SOD (superoxide dismutase) mutants of G. diazotrophicus could not reduce efficiently the ROS accumulated in the period of 1–7 h after inoculation. In addition, they measured PR genes expression and detected that expression of JA/ET pathway gene increased during wild type–plant interaction, but not to both mutants. These data suggest that G. diazotrophicus alters its redox metabolism during BNF by increasing antioxidant transcript levels to circumvent ROS inhibitory effect to nitrogenase activity. Probably this transcriptional regulatory mechanism protects nitrogenase activity during initial stages of plant colonization.
Genes homologous to those of alternative asparagine biosynthesis pathway and its role during nitrogen fixation were investigated since free asparagine, as well as others amino acids, inhibits nitrogenase activity 112, 113. Further, genome analyses revealed that genes of asparaginyl‐tRNA and asparagine synthetase orthologs are absent in the G. diazotrophicus genome. However, the correlation between repression of nifD expression and increase of Asparagine level indicated that genes for an alternative route that converts Asp‐tRNAAsn into Asn‐tRNAAsn by a glutamine‐dependent Asp‐tRNAAsn amidotransferase B, encoded by the operon gatCAB, might be present. In fact, in G diazotrophicus the presence of gatCAB operon indicates that the ORF GDI2232 encodes an Aspartyl‐tRNA synthetase of ND‐AspRS type 113. The role of asparagine and glutamine as N storage molecule in plant tissues, including sugarcane, is a general rule; however, their role in plant bacteria interaction is scarce and requires more investigation. The content of amino acids in sugarcane sap, apoplast, and symplast has been evaluated and shows that Asn is one of the most abundant 114, 115. It is known that Asn and Gln are amino acids that repress nitrogen fixation in vitro. Noteworthy, the presence of ORFs coding for putative l‐asparaginase precursor (GDI3138), l‐asparaginase II protein (GDI1250), and ORFs coding putative (aspartate) aminotransferase in G. diazotrophicus genome may represent an adaptive advantage to its endophytic behavior. Nonetheless, a detailed study is necessary to justify this assumption.
Kerby and Roberts 116 identified in G. diazotrophicus genome the presence of two ORFs coding predicted proteins similar to R. rubrum CowN (CO weal‐nitrogenase) and transcriptional regulator CooA, which belongs to the Crp/Fnr family. The ORFs GDI_3488 and GDI_3487, previously annotated as hypothetical protein 60, share common features of organization and motifs to cowN and cooA, respectively. Interestingly, the characterization of these genes in R. rubrum using PSI‐Blast searches revealed that they are widespread and generally presents similar organization in nitrogen‐fixing bacteria. The importance of CooA and CowN for Mo‐nitrogenase‐dependent functioning in the presence of CO was shown to R. rubrum and R. capsulatus, but not to the Fe‐dependent nitrogenase of the latter 116, 117. The authors suggested that CooA and CowN may act as a two component system that senses and modulates the Mo‐dependent nitrogenase activity by protecting it in the presence of CO in R. capsulatus and also in many others nitrogen fixing organisms. However, how this protection mechanism works and its relevance to nitrogen fixation is not yet understood.
Here, we presented some examples of the functional genomics studies that have already been published about G. diazotrophicus, but many others have already been thoroughly reviewed or are underway 118, 119.
Colonization
It is considered an endophytic bacterium because it has low rate of survival in the soil and was found colonizing the intercellular space of plant tissues of sugarcane 76, 77, 120, 121. This bacterium is located in different parts of the plant as described by Reis et al. 79 and James et al. 120. They demonstrated during “in vitro” inoculation studies under controlled conditions, that G. diazotrophicus enters into sugarcane micropropagated plants through the tissue of secondary roots then the bacteria penetrated inner tissues and colonize the intercellular spaces (apoplast). Other possible points of infection are wounds and the stomata of sugarcane plants 121. It also colonizes tip of roots and root hairs of other plants such as wheat, sorghum, and rice as showed using reporter genes 123, 124. At field conditions, the main route of transmission is by vegetative multiplication of stem pieces of sugarcane, although the trash should also serve as an alternative inoculum source when incorporated into the soil 79. Another possibility to introduce this bacterium in plants appears to be related to the phloem sap sucking by the insects (mealybugs) presenting this species in the lymph and living within sugarcane leaves sheath pocket 82. Caballero‐Mellado and Martínez‐Romero 83 hypothesized that these insects and also micorrhyzal spores could be responsible for local dispersion of this species within short distance while sugarcane setts and bud chips used to propagate sugarcane could carry the bacteria to further distant geographic regions. No further evidence that these insects are responsible for the dispersal of G. diazotrophicus species is reported, although it is plausible that this occurs. Unfortunately, ecological studies are underemphasized nowadays and this data is not available to a great number of newer described species.
Oliveira et al. 125 observed colonization of micropropagated sugarcane using this species in combination with four other strains of diazotrophs. Promising results were obtained when micropropagated sugarcane plants were inoculated with the type strain PAL5 in combination with small doses of nitrogen as shown by Moraes and Tauk‐Tornisielo 126. Oliveira et al. 125 showed that the combined inoculation of five endophytic diazotrophs promotes a synergistic effect when compared with the individual bacterial inoculation in micro‐propagated plants of sugarcane in pots and later at field conditions where increases of up to 30% in the accumulation of N via BNF were observed in two varieties of sugarcane planted in three soil types 127.
Plant growth‐promoting strategies
Sevilla et al. 128 described the contribution of inoculation with G. diazotrophicus in the nutrition of sugarcane and found that other factors influence on plant growth, such as growth regulators production. The ability of G. diazotrophicus to fix nitrogen and growth promotion of sugarcane was evaluated by comparing plants inoculated with the wild type (PAL5T) and an Nif mutant (MAd3A—carry a nifD mutation) in two experiments 128. Both, the type strain and the mutant, colonized sugarcane plants and persisted in mature plants. Under conditions of nitrogen deficiency, plants inoculated with PAL5T generally grew better and had a higher content of total nitrogen 60 days after planting when compared to plant inoculated with the Nif mutant. These results indicate that the transfer of fixed nitrogen from G. diazotrophicus to sugarcane may be an important mechanism for the growth promotion in this association. When nitrogen was not limiting, the stimulation of growth was also observed in plants inoculated with both bacteria suggesting an additional effect of G. diazotrophicus inoculation related to growth promotion 128. This contribution to growth was also observed by Riggis et al. 129] G. diazotrophicus was inoculated into maize plants. Among plant‐growth substances produced by G. diazotrophicus, the indole acetic acid and gibberellins A1 and A3 are phytohormones that act on plant root growth and development of aerial part tissues 130, 131.
G. diazotrophicus synthetize gluconic acid by the extracellular oxidation of the glucose by the action of the enzyme glucose dehydrogenase (GDH‐PQQ), localized in the perisplasmic space 86, 132, leading to the production of gluconic acid. This mild non‐corrosive acid can, besides lowering the pH, promote chelation and exchange reactions and has been associated with phosphate and zinc solubilisation/chelation by G. diazotrophicus 133, 134, 135, 136, 137, 138. Saravanan et al. 139 observed that G. diazotrophicus grown in Zn‐amended broth suffers deformation leading to pleomorphic, aggregate‐like cells. Noteworthy, characterization of a mutant unable to grown in the presence of Zn, Co, and Cd salts revealed that the product of czcA gene is responsible for G. diazotrophicus resistance to these heavy metals 140.
Biological control
Another promising effect of G. diazotrophicus inoculation is related to the biological control of other microrganisms, such as Xanthomonas albilineans 141, 142, Colletotrichum falcatum 143, Helminthosporium spp. 144, and Fusarium spp. 145. By cDNA‐AFLP analysis, some plant genes (using leaf tissue) involved in biocontrol activity was identified 108. These results indicate that inoculation stimulates genes involved in plant defense such as genes controlling the ethylene defense pathway. This pathway is activated when sugarcane micropropagated plants are inoculated with endophytic bacteria 146, 147, 148. Even nematodes can be controlled by inoculating G. diazotrophicus as demonstrated by Chawla et al. 149 that used the isolate number 35–47 to control Meloidogyne incognita in cotton.
Gluconacetobacter johannae and Gluconacetobacter azotocaptans
In contrast to G. diazotrophicus, which inhabits inner plant tissues as an endophyte, G. johannae and G. azotocaptans were only found colonizing the rhizosphere of coffee plants 51. Later on, G. azotocaptans was also isolated from the rhizosphere of corn 144.
Little information is available about other AAB‐N2‐fixing plant interaction. In the case of G. azotocaptans, Mehnaz and Lazarovits 144 conducted a trial inoculating plants of four varieties of maize in a greenhouse experiment using sterile soil substrate. The inoculation consisted of G. azotocaptans, Azospirillum lipoferum, and Pseudomonas putida. At 30 days after planting, the authors observed greater root growth and dry mass of the aerial part of the inoculated pots and also observed that some of the strains isolated from the rhizosphere of maize in Canada presented significant plant growth expressed as increased root/shoot mass compared with non‐inoculated plants in sand and/or soil, depending on the combination of bacteria and maize variety tested.
Nitrogen‐fixing species in Komagataeibacter genus
The genus Komagataeibacter was proposed to group members of Gluconacetobacter species that cluster closely to G. xylinus 150. Most of representatives of this new genus are known to be of industrial application but two of them are also nitrogen‐fixing bacteria.
The species Gluconacetobacter kombuchae considered a heterotypic synonym of previously named G. hansenii 48was lately reclassified as Kamagataeibacter hansenii. It was isolated during a survey of bacteria associated to Kombucha tea together with Acetobacter nitrogenifigens 52. Kombucha tea is a fermented beverage that contains an association of yeast and bacteria that takes 7–10 days to be prepared. The authors utilized aliquots of the final preparation of the tea to inoculate solid plates containing LGI medium described by Cavalcante and Döbereiner 3 but with final pH 4.5 for its isolation. This bacterium also grows in the presence of 30% of glucose or sucrose and can produce cellulose. Sequences deposited at GenBank and described as partial nifA (EF620555) and nifH (DQ141200) coding regions do not share identities/similarities to deduced amino acids of others nitrogen fixing bacteria, as indicated by blast analysis.
Komagataeibacter (Gluconacetobacter) kakiaceti was isolated by Iino et al. 62 from traditional kaki vinegar (produced from fruits of kaki, Diospyros kaki Thunb). Recently, the genome of the K. kakiaceti JCM 25156 was sequenced and revealed presence of genes homologous to nif and other regulatory proteins related to N‐metabolism. Its whole genome sequence (WGS) is available at GenBank (scaffolds accession number NZ_BAIO01000001‐NZ_BAIO01000947). However, up to date no further experimental evidence of N‐fixation by this species was reported.
Swaminathania
Swaminathania salitolerans was classified as a new genus and new species by Loganathan and Nair 53. These authors identified new isolates tolerant to salinity stress using rhizosphere, roots, and stems of mangrove‐associated wild rice plants (Porteresia coarctata Tateoka). The medium used to obtain these new isolates was a semisolid LGI culture medium without the addition of nitrogen, final pH 5.5 with the addition of 250 mM NaCl. Samples were collected in the city of Tamil Nadu in India, where 41 isolates were obtained and identified as rods, Gram‐negative, mobile, and with peritrichous flagella. Strains grew well in the presence of increasing concentrations of acetic acid (0–35%) in a very acid pH, 3.5 and also could grow in the presence of 3% NaCl and 1% KNO3. Isolates were able to fix nitrogen and solubilized phosphate in the presence of this level of salt, mimicking the location of its isolation. The colonies grown on LGI medium are initially yellow orange but become darker after aging, smooth, and raised margin, characteristics commonly found in this bacterial family. In this case, there is a description of fixing species using Acetylene Reduction Assay (ARA) to estimate nitrogenase activity during growth in semi‐solid LGI medium, besides PCR amplification of nifD. But no further works using this bacterial species were published and therefore its agricultural importance is unknown.
Asaia
The Asaia genus was first described with a single species A. bogorensis and then six more species were included: A. siamensis, A. krungthepensis, A. lannaensis, A. platycodi, A. prunellae, and A. astilbes 40, 61, 151, 152. These strains produce low quantities of acetic acid from ethanol and grew in medium with dulcitol as the sole carbon source, indicating that they belong to the genus Asaia 54. The isolates also grow on LGI containing 10% sucrose as carbon source (LGI‐P), but in this case it is acidified to pH 5.0 with acetic acid. Interestingly, this genus includes species described from samples taken from the interior of insects as mosquito Anopleles and Plasmodium that are vectors of malaria and dengue fever and cause of its sanitary importance, many studies describe the presence of various species of mosquitoes in association with this group of bacteria 153.
In India, a study was performed in order to isolate diazotrophs from three different samples: one flower called Michalia champaca, from the Anopheles mosquitoes, and from ants, such as in Tetraponera rufonigra, Pseudomyrmex, Cephalotes, and Paraponera 54, 154. It is important to emphasize that these isolates where obtained using N‐free LGI medium as described by Magalhães et al. 155 to isolate Azospirillum amazonense modifying the final pH of the medium from 6.0 to 4.5 replacing sulfuric acid by acetic acid. The same medium was used to characterize the G. diazotrophicus species in 1988 3. Samaddar et al. 54 isolated Asaia spp. as endophytes based on the surface disinfection of the plant tissue and confirmed their ability to fix nitrogen by using ARA to estimate nitrogenase activity, amplification, and sequencing of nifH‐like gene. Asaia bogorensis (MTCC 4041T), A. siamensis (MTCC 4042T), and A. platycodi AS6 strains were positive to these tests. They formed pink colonies, shiny, smooth, with entire margin in agar plates containing AG medium composed of d‐glucose (0.1%), glicerol (1.5%), peptona (0.5%), yeast extract (0.5%), malt extract (0.2%), CaCO3 (0.7%), and agar (1.5%) as described by Yamada et al. 40. This pigmentation increased after prolonged incubation at 4 °C. All of these isolates were classified as aerobic grow at pH 4.5 and 30 °C. Acetate and lactate are oxidized to carbon dioxide and water but the activity was considered low. Partial sequences of nifH‐like genes from several isolates and Asaia type strains were obtained and, as well as their genomes 63, are deposited at GenBank 54. Noteworthy, up to date blast analysis revealed that these sequences blast only among them and with several partial nifH‐like sequences from unculturable clone or chlorophyllide reductase subunit X (bchX) partial gene sequences from several microbial genome or unculturable clones (Fig. 1).
Figure 1.
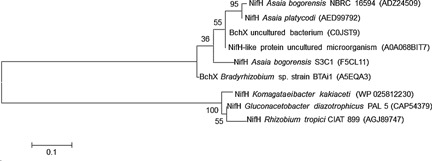
Maximum Likelihood tree of partial NifH and BchX proteins selected from protein sequences blast analysis using nifH gene deduced protein from G. diazotrophicus and Asaia bogorensis as query. Maximum Likelihood method was based on the Dayhoff matrix model in MEGA5 156. The percentage of trees in which the associated taxa clustered together is shown next to the branches (bootstrap values).
Acetobacter
Although this genus is the oldest of Acetobacteraceae family, the description of diazotrophs representatives was first raised by the description of Acetobacter diazotrophicus in the 80s. However, based on detailed taxonomic and phylogenetic studies, this species was reclassified and renamed into the genus Gluconacetobacter. No other species of the genus Acetobacter had been described as diazotrophic until 2005 when Muthukumarasamy et al. 57 presented various isolates belonging to A. peroxydans that fix nitrogen. Most of the isolates were obtained from samples of flooded rice cultivated in India but studies of nifH amplification and ARA confirmed that even in the type strain of A. peroxydans LMG 1635T these characteristics were present 57. Shortly thereafter, another study presented the description of the second species of nitrogen‐fixing Acetobacter named A. nitrogenifigens based on isolates obtained from Kombucha tea in India 52.
The nitrogen fixing Acetobacter species A. nitrogenifigens shows polar flagella similar to those of Gluconobacter 52. It also produces brown pigment and γ‐pyrone compounds, suggesting that 2‐ketogluconate and 2,5‐diketogluconate are also produced as found in Gluconobacter and Gluconacetobacter 18, 86, 157. Although claimed as positive for ARA, the A. nitrogenifigens RG1 partial nifH sequence deposited at GenBank (AY952470) do not blast with any nifH coding protein as also observed to K. hansenii (RG3).
An overview of the source of isolation and data about nitrogenase activity and nif genes to all these species are shown in Table 2.
Table 2.
Source of isolation and characterization of nitrogen fixing bacteria belonging to Acetobacteraceae family
| Genus | Species | Source of isolation | ARA | Molecular data |
|---|---|---|---|---|
| Gluconacetobacter | G. diazotrophicus | Sugarcane, roots, and stems | + | WGS |
| G. johannae | Coffee plants, rhizosphere, and rhizoplane | + | nifH sequence | |
| G. azotocaptans | + | |||
| Asaia | A. siamensis | Tropical flowers | + | nifH sequenceb |
| A. bogorensis | Flowers (B. purpurea tree and plumbago), fermented glutinous rice | + | ||
| A. platycodi | Ballon flower (Platycodongrandiflorum) | + | ||
| Swaminathania | S. salitorerans | Mangrove associated wild rice rhizosphere, roots, stems, and leaves | + | nifH amplification |
| Komagataeibacter | K. (kombuchae) hansenii | Kombucha mat suspension | n.s. | nifH sequencec |
| K. kakiaceti | Sample of vinegar suspension from kaki fruit | n.d. | nifH blast analysis | |
| Acetobacter | A. nitrogenifigens | Aliquots of Kombucha mat suspension | n.s. | nifH sequencec |
| A. peroxydans | Wetland rice varieties, rhizosphere, roots and stems | +a | nifH amplification |
n.d., not determined; n.s., data not shown.
Observed values were low and inconsistent.
Blast among Asaia spp nifH sequences but not to other Acetobacteraceae.
Agricultural applications
The agricultural application of species and strains belonging to Acetobacteraceae family will be based almost entirely in a single species, G. diazotrophicus. This species is the oldest described and characterization of its agricultural potential to important crops like sugarcane was quite widespread in Brazil and other countries. For the other nitrogen fixing species descriptions of use are rare or no report of agricultural application is available.
The use of diazotrophs in agriculture has been explored for over 30 years and its apex in the past century, the decade of 80–90, then the description of G. diazotrophicus. One of the first reports that populations of diazotrophs could be affected by increasing doses of N‐fertilizer was made by Vose et al. 158 in sugarcane which showed that high levels of mineral N caused a significant reduction in the acetylene reduction activity, very popular method which measures the indirect activity of the nitrogenase enzyme acting as a competitive inhibitor. This effect was believed to inhibit this enzyme synthesis. In 1993, after the description of G. diazotrophicus, studies conducted in Mexico by Fuentes‐Ramírez et al. 159 reported that the association between G. diazotrophicus and sugarcane could be severely limited by high N‐fertilization, which would explain the decrease in acetylene reduction activity. In their study, the crops fertilized with 120 kg N ha−1 showed higher number of isolates than the plots fertilized with 300 kg N ha−1, levels not applied in Brazil. Muthukumarasamy et al. 160, 161 obtained similar results in India for G. diazotrophicus. They suggest that this effect was not directly related to the presence of high levels of nitrogen fertilizer in sugarcane crop since this bacterium is able to grow and fix nitrogen “in vitro” in presence of high concentrations of NO3 (60 mM). It is more likely that at these high N doses the physiological state of the plant undergoes changes and subsequently influences negatively the population of this organism. Muñoz‐Rojas and Caballero‐Mellado 162 observed a negative effect on G. diazotrophicus population in the presence of high doses of nitrogen appled in sugarcane planted in Mexico. These results were confirmed by Reis Jr. et al. 163 using two sugarcane varieties planted in a sand soil fertilized with 300 kg de N ha−1 in comparison with the control without N application. Only the variety SP792312 presented plant with high levels of total N in the fertilized plots and lower numbers of G. diazotrophicus. Medeiros et al. 164 utilized different sources of nitrogen and observed that G. diazotrophicus reduced acetylene reduction activity in the presence of high levels of N.
Studies conducted in India with the application of G. diazotrophicus were repeatedly evaluated by Suman et al. 153, 154, 155. They reported that the population of G. diazotrophicus was influenced by increased doses of N‐fertilizer and that N efficiency in sugarcane increased in the presence of G. diazotrophicus inoculation in greenhouse experiments 166. Later on, Suman et al. utilized one strain of G. diazotrophicus, named IS100, besides strains of A. brasilense and Azotobacter chrococcum to evaluate nitrogen efficiency applied in increased doses on sugarcane planted in India 167. G. diazotrophicus showed the best results of crop yield, followed by its combination with A. chrococcum and A. brasilense.
Application of G. diazotrophicus was also evaluated in the germination of stem pieces of sugarcane by De la Cruz et al. 168 in Philippines. These authors tested inoculation with different cell densities (108, 1010, and 1012 cells ml−1) and methods of application (spray, immersion for 2 h and dipping during 2 min). They observed that inoculation led to increase in percentage survival plant height and shoot/root biomass when compared to the control at 45 days after planting. Introduction of microbial inoculant in 1012 ml−1 cells by immersion method produced taller plants with greater biomass and root compared to other treatments and uninoculated control.
Strains of Gluconacetobacter diazotrophicus, Azospirillum amazonense, Herbaspirillum seropedicae, Herbaspirillum rubrisubalbicans, and Burkholderia tropica species were applied in sugarcane using pots filled with 60 kg of soil and also field experiments planted in three different soil types in São Paulo and Rio de Janeiro states of Brazil showing contributions of the biological process with higher crop yields of different varieties SP70‐1143, SP81‐3250, RB72454, RB867515 125, 127, 169, 170. Oliveira et al. 127 used the technique of δ 15N (natural abundance of 15N in the soil) and tested seven types of inoculants and found that the inoculant containing five strains described above showed the best results. These authors also quantitated the contribution of BNF showing that the mixture of five strains obtained 29.2% of the accumulated N derived from the air. Schultz et al. 170 utilized the same five strains and modifications of the δ 15N method of BNF quantification of soil applied in the sugarcane yield of RB72454 and RB867515 varieties and showed that plant biomass increased, but found no contributions of nitrogen fixation process by the inoculation. In order to understant how the sugarcane was colonized by this mixture, a fluorescent in situ hybridization (FISH) analysis based on rRNA‐targeted oligonucleotide probes confirmed that in micropropagated sugarcane inoculated with this mixture of five species reached the endophytic habitat of micropropagated sugarcane plantlets through active infection of the root cap and emerging zone of secondary roots, although with different efficiencies due to apparently different competitiveness for colonization 133.
Maheshkumar et al. 133 observed that this species was able to solubilize rock phosphate “in vitro,” and it could be one of several effects that can promote plant growth after inoculation. Sugar beet has also been used to check the response of G. diazotrophicus inoculation as described by Jambukar and Wange 155. In 2009, Tian et al. 171 observed the effect of G. diazotrophicus inoculation in different maize genotypes, 17 hybrids, and 10 sweet corn varieties planted in Canada. Colonization of 11 hybrids and 9 sweet corn varieties by G. diazotrophicus was confirmed using species specific primers, but populations were quantified only in the order of 200–3000 cells g−1 of plant tissue.
G. diazotrophicus is known worldwide for nitrogen fixation but this is only one of its mechanisms of interest for agriculture and other industrial processes. For example, G. diazotrophicus strain SRT4 has genes for the production of levan‐sucrase both endo and exo levanases which are expressed under stress conditions 172, 173. Another product of its growth is bacteriocins that may act to control growth of other microorganisms or even other strains of the same species 174. This bacteriocin is constitutively expressed in different conditions of culture medium and dependent on the strain tested.
However, is there G. diazotrophicus as commercial product for use in agriculture? The answer is yes. Descriptions of products containing G. diazotrophicus can be found elsewhere. In Argentina, the ene‐2 Endophyte‐Plus® sold by the company ARBO SRL Laboratory, is recommended to be applied as an inoculant for wheat, maize, soybean, and tomato (http://www.arbolab.com.ar/es/productos/2lvl/prom.html). In Mexico, the company Agro Organics GAIA sells a product containing a mixture of G. diazotrophicus and the fungi Penicillium called Glubac® (http://www.organicosgaia.com.mx/biotransferentes-de-nutrientes.html).
In Brazil, origin of the G. diazotrophicus description, a patent based on a microorganism species or strain is not allowed, but to several other countries it is. In the United States, there is a patent for use of several nitrogen fixing bacteria, including G. diazotophicus, to enhance plant growth in cereals (US 7393678 B2), and other claiming its use to reduce N fertilization in sugar rich plants, especially sugar beet (20110225679), showing that it can be part of a product for agricultural use. In Brazil, bioprocess can be a matter of patent claims, such as the growth conditions of this bacterial to the production of biomass and fermentation products (PI0917666‐7 A2).
However, a good product for the industry needs to possess a long shelf‐life in order to reduce the costs and facilitates the distribution. Unfortunately, a few studies have developed vehicles and protective substances that increase the longevity of cells of this species. Nita et al. 175 tested several substances such as cell protective for G. diazotrophicus under different temperatures (4 and 25 °C). Efficacy was evaluated in tests of wheat inoculation under greenhouse and field conditions. The best method tested was the application of molasses (cane syrup) with 0.1% (w/v) of NH4Cl. Trehalose, Arabic gum, and Polyethylene glycol 300 (PEG 300) presented the best results. Addition of l‐ascorbic acid (0.02% w/v) to the preservation medium also enhanced the efficacy of the substances used as protectors. After 8–9 months of stock ay 4 °C, G. diazotrophicus (strain L1) showed the best results of shelflife in the presence of arabic gum (5% w/v) and PEG 300 (5% w/v), respectively, and also keeping the growth promotion effect. Silva et al. 176 tested a polymer based on carboxymethylcellulose on the survival of G. diazotrophicus strain PAL5T with a shelf life of 109 cell ml−1 for 120 days.
To date, data about agricultural application of other nitrogen fixing Acetobacteraceae are restricted to Asaia and it is based on a single report of Weber et al. in 2010 177 that utilized a single strain of A. bogorensis (AB 219) as inoculant for pineapple and monitored colonization by using agar plates of JNFb medium (malate as a carbon source and final pH 5.8) and population by the Most Probable Number (NMP). The growth and fruiting of pineapple were benefited from the inoculation of A. bogorensis (strain 219) associated with irrigation and increasing doses of organic fertilizer (compost).
Final considerations
Since the discovery of Gluconacetobacter diazotrophicus in 1988, many other diazotrophs belonging to the family Acetobacteraceae were described as nitrogen fixing species, but this number can increase. The strategy to isolate and identify new species generally is not based on criteria of biological nitrogen fixation ability and this character is not a discriminatory one. Interestingly, we observed that some researcher groups used the nitrogen free LGI medium as a strategy to obtain new isolates. It is expected that using N‐free medium during isolation process can enrich populations of nitrogen fixing bacteria leading to recovery of many of them from environmental samples. In addition, the original pH of LGI was 5.5, but several new species were described with a simple modification of the final pH to levels lower than 5.0. Since representatives of this family are adapted to acidic environment, lowering pH can be considered another strategy used by many authors to isolate and describe new species of nitrogen fixing Acetobacteraceae.
It is noteworthy that as many as new species have been described over the last 25 years, publications containing studies of their ecology and distribution diminished considerably. G. diazotrophicus is the most studied nitrogen fixing bacteria of this family for agricultural application. Since the beginning, several studies describing its survival, habitats, mode of plant colonization, and transference to new hosts have accumulated in the literature. Based on them, the description of G. diazotrophicus as a true endophyte was proposed and accepted. The endophytic behavior of G. diazotrophicus is based on many ecological surveys and studies while these data are lacking to other species described. Actually, nowadays publications of ecological research are an exception when we compare with the increasing numbers of species description mainly based on a set of physiological and molecular data, small numbers of specimens or even only one representative.
Nitrogen fixation is a biological processes well characterized and understood, at to some points, in pure culture and in vitro that occurs when an appropriate energy source is available in combination with the optimal temperature, pH and controlled O2 concentration. Nonetheless, it is not an easy task to really prove that a single strain is responsible for part of the assimilated nitrogen in plant, especially under field conditions.
In general, the main effects that are easily identified in plants that establish association with non‐symbiotic nitrogen fixing bacteria are root surface enhancement, increased grain production, and early maturation. Besides nitrogen fixation ability, G. diazotrophicus also produces growth hormones such as auxins and gibberellins and also can be considered PGPB when compared to A. brasilense. In agricultural perspective the application of Acetobacteraceae species can contribute to plant growth and improve nitrogen assimilation of the host plant. However, under field conditions the effect of the number of bacteria in the plant versus the contribution of biological nitrogen fixation and/or plant growth promotion is not clearly established. It is already reported that introduced populations can undergo changes not only on their physiological aspects, but also in genomic aspects. So far, over more than 30 years of studies most of the knowledge of these aspects of the bacterium–plant interaction is based on analysis conducted under controlled laboratory conditions.
Although the centennial knowledge of this versatile bacterial family to industrial application, a lot has to be done about the potential of the nitrogen fixing Acetobacteracea to agricultural application and even to many other industrial biotechnological processes is limited yet. To improve agricultural use or even to broaden the industrial purpose of these nitrogen fixing Acetobacteraceae species depends on development of new biotechnological data.
For development of new biotechnological application and products, it will be necessary to increase knowledge and exploit the genomic potential for adaptation, competition, and survival of these bacteria. Introdution of certain species to different plants and/or environmental conditions has to be explored. Besides, the demand of development of methods, easily applied under field conditions, for bacterial inoculation, monitoration, and validation must be constantly considered. In addition, further efforts to characterize the ecology related to these microorganisms and plant relationship is essential. Selection or development of genetically modified bacteria adapted to field competition, stress, and interaction with other components of the microbiota will be one of the goals to improve the inoculation technology worldwide.
Acknowledgments
The authors wish to thank the Coordination of Improvement of Higher Education Personnel—CAPES, the National Council for Scientific and Technological Development—CNPq and the Carlos Chagas Foundation for Research Support of the State of Rio de Janeiro—FAPERJ for the scholarships. Finantial support and also scholarships from CNPq, project number 303125/2013‐6 and also CNPq/INCT‐FBN (Process No. 573828/2008‐3).
References
- 1. Pasteur, L. , 1864. Mémoire sur la fermentation acétique. Ann. Sci. LÉcole Norm. Supér. Paris, 1, 113–158. [Google Scholar]
- 2. Beijerinck, M.W. , 1898. Über die Arten der Essigbakterien. Zbl. Bakteriol. Parasitenkunde Infekt. Hyg., 4, 209–216). [Google Scholar]
- 3. Cavalcante, V.A. , Döbereiner, J. , 1988. A new acid‐tolerant nitrogen‐fixing bacterium associated with sugarcane. Plant Soil, 108, 23–31. [Google Scholar]
- 4. Gillis, M. , De Ley, J. , 1980. Intra‐and intergeneric similarities of the ribosomal ribonucleic acid cistrons of Acetobacter and Gluconobacter . Int. J. Syst. Bacteriol., 30, 7–27. [Google Scholar]
- 5. Kersters, K. , Lisdiyanti, P. , Komagata, K. , Swings, J. , 2006. The family Acetobacteraceae: the Genera Acetobacter, Acidomonas, Asaia, Gluconacetobacter, Gluconobacter, and Kozakia , in: Dworkin, M. , Falkow, S. , Rosenberg, E. , Schleifer, K.‐H. , Stackebrandt, E. (Eds.), The Prokaryotes, Springer, New York, 163–200. [Google Scholar]
- 6. Raspor, P. , Goranovič, D. , 2008. Biotechnological applications of acetic acid bacteria. Crit. Rev. Biotechnol., 28, 101–124. [DOI] [PubMed] [Google Scholar]
- 7. Rihs, J.D. , Brenner, D.J. , Weaver, R.E. , Steigerwalt, A.G. et al., 1993. Roseomonas, a new genus associated with bacteremia and other human infections. J. Clin. Microbiol., 31, 3275–3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Greenberg, D.E. , Ding, L. , Zelazny, A.M. , Stock, F. et al., 2006. A novel bacterium associated with lymphadenitis in a patient with chronic granulomatous disease. PLoS Pathog., 2, e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Greenberg, D.E. , Porcella, S.F. , Stock, F. , Wong, A. et al., 2006. Granulibacter bethesdensis gen. nov., sp. nov., a distinctive pathogenic acetic acid bacterium in the family Acetobacteraceae . Int. J. Syst. Evol. Microbiol., 56, 2609–2616. [DOI] [PubMed] [Google Scholar]
- 10. Tuuminen, T. , Roggenkamp, A. , Vuopio‐Varkila, J. , 2007. Comparison of two bacteremic Asaia bogorensis isolates from Europe. Eur. J. Clin. Microbiol. Infect. Dis., 26, 523–524. [DOI] [PubMed] [Google Scholar]
- 11. Gouby, A. , Teyssier, C. , Vecina, F. , Marchandin, H. et al., 2007. Acetobacter cibinongensis bacteremia in human. Emerg. Infect. Dis., 13, 784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bittar, F. , Reynaud‐Gaubert, M. , Thomas, P. , Boniface, S. et al., 2008. Acetobacter indonesiensis pneumonia after lung transplant. Emerg. Infect. Dis., 14, 997–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yamada, Y. , Yukphan, P. , 2008. Genera and species in acetic acid bacteria. Int. J. Food Microbiol., 125, 15–24. [DOI] [PubMed] [Google Scholar]
- 14. Asai, T. , 1935. Taxonomic studies on acetic acid bacteria and allied oxidative bacteria isolated from fruits. A new classification of the oxidative bacteria. J. Agric. Chem. Soc. Jpn., 11, 674–708. [Google Scholar]
- 15. Asai, T. , Iizuka, H. , Komagata, K. , 1964. The flagellation and taxonomy of genera Gluconobacter and Acetobacter with reference to the existence of intermediate strains. J. Gen. Appl. Microbiol., 10, 95–126. [Google Scholar]
- 16. Frateur, J. , 1950. Essai sur la systematique des acetobacters. Cellule, 53, 287–392. [Google Scholar]
- 17. Yamada, Y. , Kondo, K. , 1984. Gluconoacetobacter, a new subgenus comprising the acetate‐oxidizing acetic acid bacteria with ubiquinone‐10 in the genus Acetobacter . J. Gen. Appl. Microbiol., 30, 297–303. [Google Scholar]
- 18. Yamada, Y. , Hoshino, K. , Ishikawa, T. , 1997. The phylogeny of acetic acid bacteria based on the partial sequences of 16S ribosomal RNA: the elevation of the subgenus Gluconoacetobacter to the generic level. Biosci. Biotechnol. Biochem., 61, 1244–1251. [DOI] [PubMed] [Google Scholar]
- 19. Yamada, Y. , Hoshino, K. , Ishikawa, T. , 1998. Validation list no. 64: validation of publication of new names and new combinations previously effectively published outside the IJSB. Int. J. Syst. Bacteriol., 48, 327–328. [DOI] [PubMed] [Google Scholar]
- 20. Parte, A.C. , 2014. LPSN‐list of prokaryotic names with standing in nomenclature. Nucleic Acids Res., 42, D613–D616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vu, H.T.L. , Yukphan, P. , Chaipitakchonlatarn, W. , Malimas, T. et al., 2013. Nguyenibacter vanlangensis gen. nov., sp. nov., an unusual acetic acid bacterium in the α‐Proteobacteria. J. Gen. Appl. Microbiol., 59, 153–166. [DOI] [PubMed] [Google Scholar]
- 22. Okamura, K. , Hisada, T. , Kanbe, T. , Hiraishi, A. , 2009. Rhodovastum atsumiense gen. nov., sp. nov., a phototrophic alphaproteobacterium isolated from paddy soil. J. Gen. Appl. Microbiol., 55, 43–50. [DOI] [PubMed] [Google Scholar]
- 23. Qu, J.‐H. , Qu, J.‐Y. , He, X.‐B. , Li, H.‐F. et al., 2013. Sediminicoccus rosea gen. nov., sp. nov., isolated from the sediment of a eutrophic lake. J. Gen. Appl. Microbiol., 59, 463–468. [DOI] [PubMed] [Google Scholar]
- 24. Malimas, T. , Chaipitakchonlatarn, W. , Vu, H.T.L. , Yukphan, P. et al., 2013. Swingsia samuiensis gen. nov., sp. nov., an osmotolerant acetic acid bacterium in the α‐Proteobacteria. J. Gen. Appl. Microbiol., 59, 375–384. [DOI] [PubMed] [Google Scholar]
- 25. Imhoff, J.F. , Trüper, H.G. , Pfennig, N. , 1984. Rearrangement of the species and genera of the phototrophic “purple nonsulfur bacteria”. Int. J. Syst. Bacteriol., 34, 340–343. [Google Scholar]
- 26. Harrison, A.P. , 1981. Acidiphilium cryptum gen. nov., sp. nov., heterotrophic bacterium from acidic mineral environments. Int. J. Syst. Bacteriol., 31, 327–332. [Google Scholar]
- 27. Hiraishi, A. , Matsuzawa, Y. , Kanbe, T. , Wakao, N. , 2000. Acidisphaera rubrifaciens gen. nov., sp. nov., an aerobic bacteriochlorophyll‐containing bacterium isolated from acidic environments. Int. J. Syst. Evol. Microbiol., 50, 1539–1546. [DOI] [PubMed] [Google Scholar]
- 28. Garrity, G. , Bergey's Manual® of Systematic Bacteriology: Volume Two: The Proteobacteria, Part A: Introductory Essays. Springer, New York, NY, 2006. [Google Scholar]
- 29. Sanchez‐Porro, C. , Gallego, V. , Busse, H.‐J. , Kampfer, P. et al., 2009. Transfer of Teichococcus ludipueritiae and Muricoccus roseus to the genus Roseomonas, as Roseomonas ludipueritiae comb. nov. and Roseomonas rosea comb. nov., respectively, and emended description of the genus Roseomonas . Int. J. Syst. Evol. Microbiol., 59, 1193–1198. [DOI] [PubMed] [Google Scholar]
- 30. Yurkov, V , Stackebrandt, E. , Holmes, A. , Fuerst, JA et al., 1994. Phylogenetic positions of novel aerobic, bacteriochlorophyll a‐containing bacteria and description of Roseococcus thiosulfatophilus gen. nov., sp. nov., Erythromicrobium ramosum gen. nov. sp. nov., and Erythrobacter litoralis sp. nov. Int. J. Syst. Bacteriol., 44, 427–434. [DOI] [PubMed] [Google Scholar]
- 31. Saitoh, S. , Suzuki, T. , Nishimura, Y. , 1998. Proposal of Craurococcus roseus gen. nov., sp. nov. and Paracraurococcus ruber gen. nov., sp. nov., novel aerobic bacteriochlorophyll a‐containing bacteria from soil. Int. J. Syst. Bacteriol., 48, 1043–1047. [DOI] [PubMed] [Google Scholar]
- 32. Alarico, S. , Rainey, F.A. , Empadinhas, N. , Schumann, P. et al., 2002. Rubritepida flocculans gen. nov., sp. nov., a new slightly thermophilic member of the α‐1 Subclass of the Proteobacteria . Syst. Appl. Microbiol., 25, 198–206. [DOI] [PubMed] [Google Scholar]
- 33. Margesin, R. , Zhang, D.‐C. , 2013. Humitalea rosea gen. nov., sp. nov., an aerobic bacteriochlorophyll‐containing bacterium of the family Acetobacteraceae isolated from soil. Int. J. Syst. Evol. Microbiol., 63, 1411–1416. [DOI] [PubMed] [Google Scholar]
- 34. Kishimoto, N. , Kosako, Y. , Tano, T. , 1993. Acidiphilium aminolytica sp. nov.: an acidophilic chemoorganotrophic bacterium isolated from acidic mineral environment. Curr. Microbiol., 27, 131–136. [DOI] [PubMed] [Google Scholar]
- 35. Kishimoto, N. , Kosako, Y. , Wakao, N. , Tano, T. , Hiraishi, A. , 1995. Transfer of Acidiphilium facilis and Acidiphilium aminolytica to the genus Acidocella gen. nov., and emendation of the genus Acidiphilium . Syst. Appl. Microbiol., 18, 85–91. [Google Scholar]
- 36. Vasilyeva, L.V. , 1985. Stella, a new genus of soil prosthecobacteria, with proposals for Stella humosa sp. nov. and Stella vacuolata sp. nov. Int. J. Syst. Bacteriol., 35, 518–521. [Google Scholar]
- 37. Fritz, I. , 2004. Phylogenetic relationships of the genera Stella, Labrys and Angulomicrobium within the “Alphaproteobacteria” and description of Angulomicrobium amanitiforme sp. nov. Int. J. Syst. Evol. Microbiol., 54, 651–657. [DOI] [PubMed] [Google Scholar]
- 38. Urakami, T. , Tamaoka, J. , Suzuki, K.‐I. , Komagata, K. , 1989. Acidomonas gen. nov., incorporating Acetobacter methanolicus as Acidomonas methanolica comb. nov. Int. J. Syst. Bacteriol., 39, 50–55. [Google Scholar]
- 39. Yamashita, S.‐I , Ushimura, T. , Komagata, K. , 2004. Emendation of the genus Acidomonas Urakami, Tamaoka, Suzuki and Komagata 1989. Int. J. Syst. Evol. Microbiol., 54, 865–870. [DOI] [PubMed] [Google Scholar]
- 40. Yamada, Y. , Katsura, K. , Kawasaki, H. , Widyastuti, Y. et al., 2000. Asaia bogorensis gen. nov., sp. nov., an unusual acetic acid bacterium in the alpha‐Proteobacteria. Int. J. Syst. Evol. Microbiol., 50, 823–829. [DOI] [PubMed] [Google Scholar]
- 41. Yukphan, P. , Malimas, T. , Potacharoen, W. , Tanasupawat, S. et al., 2005. Neoasaia chiangmaiensis gen. nov., sp. nov., a novel osmotolerant acetic acid bacterium in the α‐Proteobacteria. J. Gen. Appl. Microbiol., 51, 301–311. [DOI] [PubMed] [Google Scholar]
- 42. Kämpfer, P. , Andersson, M.A. , Jäckel, U. , Salkinoja‐Salonen, M. , 2003. Teichococcus ludipueritiae gen. nov. sp. nov., and Muricoccus roseus gen. nov. sp. nov. representing two new genera of the α‐1 subclass of the Proteobacteria. Syst. Appl. Microbiol., 26, 23–29. [DOI] [PubMed] [Google Scholar]
- 43. Jojima, Y. , 2004. Saccharibacter floricola gen. nov., sp. nov., a novel osmophilic acetic acid bacterium isolated from pollen. Int. J. Syst. Evol. Microbiol., 54, 2263–2267. [DOI] [PubMed] [Google Scholar]
- 44. Belova, S.E. , Pankratov, T.A. , Detkova, E.N. , Kaparullina, E.N. et al., 2009. Acidisoma tundrae gen. nov., sp. nov. and Acidisoma sibiricum sp. nov., two acidophilic, psychrotolerant members of the Alphaproteobacteria from acidic northern wetlands. Int. J. Syst. Evol. Microbiol., 59, 2283–2290. [DOI] [PubMed] [Google Scholar]
- 45. Yukphan, P. , Malimas, T. , Muramatsu, Y. , Potacharoen, W. et al., 2011. Neokomagataea gen. nov., with descriptions of Neokomagataea thailandica sp. nov. and Neokomagataea tanensis sp. nov., osmotolerant acetic acid bacteria of the α‐Proteobacteria. Biosci. Biotechnol. Biochem., 75, 419–426. [DOI] [PubMed] [Google Scholar]
- 46. Ramirez‐Bahena, M.H. , Tejedor, C. , Martin, I. , Velazquez, E. et al., 2013. Endobacter medicaginis gen. nov., sp. nov., isolated from alfalfa nodules in an acidic soil. Int. J. Syst. Evol. Microbiol., 63, 1760–1765. [DOI] [PubMed] [Google Scholar]
- 47. Dutta, D. , Gachhui, R. , 2007. Nitrogen‐fixing and cellulose‐producing Gluconacetobacter kombuchae sp. nov., isolated from Kombucha tea. Int. J. Syst. Evol. Microbiol., 57, 353–357. [DOI] [PubMed] [Google Scholar]
- 48. Cleenwerck, I. , De Wachter, M. , Gonzalez, A. , De Vuyst, L. et al., 2009. Differentiation of species of the family Acetobacteraceae by AFLP DNA fingerprinting: Gluconacetobacter kombuchae is a later heterotypic synonym of Gluconacetobacter hansenii . Int. J. Syst. Evol. Microbiol., 59, 1771–1786. [DOI] [PubMed] [Google Scholar]
- 49. Yamada, Y. , Yukphan, P. , Vu, H.T.L. , Muramatsu, Y. et al., 2012. Subdivision of the genus Gluconacetobacter Yamada, Hoshino and Ishikawa 1998: the proposal of Komagatabacter gen. nov., for strains accommodated to the Gluconacetobacter xylinus group in the α‐Proteobacteria. Ann. Microbiol., 62, 849–859. [Google Scholar]
- 50. Yamada, Y. , Yukphan, P. , Vu, H.L. , Muramatsu, Y. et al., 2012. Description of Komagataeibacter gen. nov., with proposals of new combinations (Acetobacteraceae). J Gen Appl. Microbiol., 58, 397–404. [DOI] [PubMed] [Google Scholar]
- 51. Fuentes‐Ramírez, L.E. , Bustillos‐Cristales, R. , Tapia‐Hernández, A. , Jiménez‐Salgado, T. et al., 2001. Novel nitrogen‐fixing acetic acid bacteria, Gluconacetobacter johannae sp. nov. and Gluconacetobacter azotocaptans sp. nov., associated with coffee plants. Int. J. Syst. Evol. Microbiol., 51, 1305–1314. [DOI] [PubMed] [Google Scholar]
- 52. Dutta, D. , Gachhui, R. , 2006. Novel nitrogen‐fixing Acetobacter nitrogenifigens sp. nov., isolated from Kombucha tea. Int. J. Syst. Evol. Microbiol., 56, 1899–1903. [DOI] [PubMed] [Google Scholar]
- 53. Loganathan, P. , Nair, S. , 2004. Swaminathania salitolerans gen. nov., sp. nov., a salt‐tolerant, nitrogen‐fixing and phosphate‐solubilizing bacterium from wild rice (Porteresia coarctata Tateoka). Int. J. Syst. Evol. Microbiol., 54, 1185–1190. [DOI] [PubMed] [Google Scholar]
- 54. Samaddar, N. , Paul, A. , Chakravorty, S. , Chakraborty, W. et al., 2011. Nitrogen fixation in Asaia sp. (family Acetobacteraceae). Curr. Microbiol., 63, 226–231. [DOI] [PubMed] [Google Scholar]
- 55. Yamada, Y. , 2014. Transfer of Gluconacetobacter kakiaceti, Gluconacetobacter medellinensis and Gluconacetobacter maltaceti to the genus Komagataeibacter as Komagataeibacter kakiaceti comb. nov., Komagataeibacter medellinensis comb. nov. and Komagataeibacter maltaceti comb. nov. Int. J. Syst. Evol. Microbiol., 64, 1670–1672. [DOI] [PubMed] [Google Scholar]
- 56. Lisdiyanti, P. , Katsura, K. , Potacharoen, W. , Navarro, R.R. et al., 2003. Diversity of acetic acid bacteria in Indonesia, Thailand, and the Philippines. Microbiol. Cult. Coll., 19, 91–98. [Google Scholar]
- 57. Muthukumarasamy, R. , Cleenwerck, I. , Revathi, G. , Vadivelu, M. et al., 2005. Natural association of Gluconacetobacter diazotrophicus and diazotrophic Acetobacter peroxydans with wetland rice. Syst. Appl. Microbiol., 28, 277–286. [DOI] [PubMed] [Google Scholar]
- 58. Katsura, K. , Kawasaki, H. , Potacharoen, W. , Saono, S. et al., 2001. Asaia siamensis sp. nov., an acetic acid bacterium in the alpha‐proteobacteria. Int. J. Syst. Evol. Microbiol., 51, 559–563. [DOI] [PubMed] [Google Scholar]
- 59. Meenakshisundaram, M. , Santhaguru, K. , 2010. Isolation and nitrogen fixing efficiency of a novel endophytic diazotroph Gluconacetobacter diazotrophicus associated with Saccharum officinarum from southern districts of Tamilnadu. Int. J. Biol. Med. Res., 1, 298–300. [Google Scholar]
- 60. Bertalan, M. , Albano, R. , de Pádua, V. , Rouws, L. et al., 2009. Complete genome sequence of the sugarcane nitrogen‐fixing endophyte Gluconacetobacter diazotrophicus PAL5. BMC Genomics, 10, 450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Suzuki, R. , Zhang, Y. , Iino, T. , Kosako, Y. et al., 2010. Asaia astilbes sp. nov., Asaia platycodi sp. nov., and Asaia prunellae sp. nov., novel acetic acid bacteria isolated from flowers in Japan. J. Gen. Appl. Microbiol., 56, 339–346. [DOI] [PubMed] [Google Scholar]
- 62. Iino, T. , Suzuki, R. , Tanaka, N. , Kosako, Y. et al., 2012. Gluconacetobacter kakiaceti sp. nov., an acetic acid bacterium isolated from a traditional Japanese fruit vinegar. Int. J. Syst. Evol. Microbiol., 62, 1465–1469. [DOI] [PubMed] [Google Scholar]
- 63. Shane, J.L. , Bongio, N.J. , Favia, G. , Lampe, D.J. , 2014. Draft genome sequence of Asaia sp. strain SF2. 1, an important member of the microbiome of Anopheles mosquitoes. Genome Announc., 2, e01202‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Lipman, J.G. , 1904. Soil bacteriological studies. Further contributions to the physiology and morphology of the members of the Azotobacter group. Rep. N. J. State Agric. Exp. Stn., 25, 237–289. [Google Scholar]
- 65. Sievers, M. , Sellmer, S. , Teuber, M. , 1992. Acetobacter europaeus sp. nov., a main component of industrial vinegar fermenters in Central Europe. Syst. Appl. Microbiol., 15, 386–392. [Google Scholar]
- 66. Franke, I.H. , Fegan, M. , Hayward, C. , Leonard, G. et al., 1999. Description of Gluconacetobacter sacchari sp. nov., a new species of acetic acid bacterium isolated from the leaf sheath of sugar cane and from the pink sugar‐cane mealy bug. Int. J. Syst. Evol. Microbiol., 49, 1681–1693. [DOI] [PubMed] [Google Scholar]
- 67. Dellaglio, F. , 2005. Description of Gluconacetobacter swingsii sp. nov. and Gluconacetobacter rhaeticus sp. nov., isolated from Italian apple fruit. Int. J. Syst. Evol. Microbiol., 55, 2365–2370. [DOI] [PubMed] [Google Scholar]
- 68. Tazato, N. , Nishijima, M. , Handa, Y. , Kigawa, R. et al., 2012. Gluconacetobacter tumulicola sp. nov. and Gluconacetobacter asukensis sp. nov., isolated from the stone chamber interior of the Kitora Tumulus. Int. J. Syst. Evol. Microbiol., 62, 2032–2038. [DOI] [PubMed] [Google Scholar]
- 69. Nishijima, M. , Tazato, N. , Handa, Y. , Tomita, J. et al., 2013. Gluconacetobacter tumulisoli sp. nov., Gluconacetobacter takamatsuzukensis sp. nov. and Gluconacetobacter aggeris sp. nov., isolated from Takamatsuzuka Tumulus samples before and during the dismantling work in 2007. Int. J. Syst. Evol. Microbiol., 63, 3981–3988. [DOI] [PubMed] [Google Scholar]
- 70. Lisdiyanti, P. , 2006. Reclassification of Gluconacetobacter hansenii strains and proposals of Gluconacetobacter saccharivorans sp. nov. and Gluconacetobacter nataicola sp. nov. Int. J. Syst. Evol. Microbiol., 56, 2101–2111. [DOI] [PubMed] [Google Scholar]
- 71. Castro, C. , Cleenwerck, I. , Trcek, J. , Zuluaga, R. et al., 2013. Gluconacetobacter medellinensis sp. nov., cellulose‐ and non‐cellulose‐producing acetic acid bacteria isolated from vinegar. Int. J. Syst. Evol. Microbiol., 63, 1119–1125. [DOI] [PubMed] [Google Scholar]
- 72. Slapšak, N. , Cleenwerck, I. , De Vos, P. , Trček, J. , 2013. Gluconacetobacter maltaceti sp. nov., a novel vinegar producing acetic acid bacterium. Syst. Appl. Microbiol., 36, 17–21. [DOI] [PubMed] [Google Scholar]
- 73. Yamada, Y. , Yukphan, P. , 2008. Genera and species in acetic acid bacteria. Int. J. Food Microbiol., 125, 15–24. [DOI] [PubMed] [Google Scholar]
- 74. Cleenwerck, I. , De Vos, P. , De Vuyst, L. , 2010. Phylogeny and differentiation of species of the genus Gluconacetobacter and related taxa based on multilocus sequence analyses of housekeeping genes and reclassification of Acetobacter xylinus subsp. sucrofermentans as Gluconacetobacter sucrofermentans (Toyosaki et al. 1996) sp. nov. , comb. nov. Int. J. Syst. Evol. Microbiol., 60, 2277–2283. [DOI] [PubMed] [Google Scholar]
- 75. Gillis, M. , Kersters, K. , Hoste, B. , Janssens, D. et al., 1989. Acetobacter diazotrophicus sp. nov., a nitrogen‐fixing acetic acid bacterium associated with sugarcane. Int. J. Syst. Bacteriol., 39, 361–364. [Google Scholar]
- 76. Dong, Z. , Canny, M.J. , McCully, M.E. , Roboredo, M.R. et al., 1994. A nitrogen‐fixing endophyte of sugarcane stems (a new role for the apoplast). Plant Physiol., 105, 1139–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Baldani, J. , Caruso, L. , Baldani, V.L. , Goi, S.R. et al., 1997. Recent advances in BNF with non‐legume plants. Soil Biol. Biochem., 29, 911–922. [Google Scholar]
- 78. Youssef, H.H. , Fayez, M. , Monib, M. , Hegazi, N. , 2004. Gluconacetobacter diazotrophicus: a natural endophytic diazotroph of Nile Delta sugarcane capable of establishing an endophytic association with wheat. Biol. Fertil. Soils, 39, 391–397. [Google Scholar]
- 79. Reis, V.M. , Olivares, F.L. , Döbereiner, J. , 1994. Improved methodology for isolation of Acetobacter diazotrophicus and confirmation of its endophytic habitat. World J. Microbiol. Biotechnol., 10, 401–405. [DOI] [PubMed] [Google Scholar]
- 80. Jimenez‐Salgado, T. , Fuentes‐Ramirez, L.E. , Tapia‐Hernandez, A. , Mascarua‐Esparza, M.A. et al., 1997. Coffea arabica L., a new host plant for Acetobacter diazotrophicus, and isolation of other nitrogen‐fixing acetobacteria. Appl. Environ. Microbiol., 63, 3676–3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Tapia‐Hernandez, A. , Bustillos‐Cristales, M.R. , Jimenez‐Salgado, T. , Caballero‐Mellado, J. et al., 2000. Natural endophytic occurrence of Acetobacter diazotrophicus in pineapple plants. Microb. Ecol., 39, 49–55. [DOI] [PubMed] [Google Scholar]
- 82. Ashbolt, N.J. , Inkerman, P.A. , 1990. Acetic acid bacterial biota of the pink sugar cane mealybug, Saccharococcus sacchari, and its environs. Appl. Environ. Microbiol., 56, 707–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Caballero‐Mellado, J. , Martinez‐Romero, E. , 1994. Limited genetic diversity in the endophytic sugarcane bacterium Acetobacter diazotrophicus . Appl. Environ. Microbiol., 60, 1532–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Reis, V.M. , Olivares, F.L. , de Oliveira, A.L.M. , dos Reis Junior, F.B. et al., 1999. Technical approaches to inoculate micropropagated sugar cane plants were Acetobacter diazotrophicus . Plant Soil, 206, 205–211. [Google Scholar]
- 85. Reis, V.M. , Döbereiner, J. , 1998. Effect of high sugar concentration on nitrogenase activity of Acetobacter diazotrophicus . Arch. Microbiol., 171, 13–18. [DOI] [PubMed] [Google Scholar]
- 86. Stephan, M.P. , Oliveira, M. , Teixeira, K.R.S. , Martinez‐Drets, G. et al., 1991. Physiology and dinitrogen fixation of Acetobacter diazotrophicus . FEMS Microbiol. Lett., 77, 67–72. [Google Scholar]
- 87. Teixeira, K.R.S. , Galler, R. , Kennedy, C. , Baldani, J.I. , 1994. Plasmid contents and nif genes detection in Acetobacter diazotrophicus strains, in: Hegazi, N. , Fayez, M. , Monib, M. (Eds.), 6th Int. Symp. Nitrogen Fixat. Non‐Legum. Ismailia Egypt, American University in Cairo Press, Cairo, 273–281. [Google Scholar]
- 88. Sevilla, M. , Meletzus, D. , Teixeira, K. , Lee, S. et al., 1997. Analysis of nif and regulatory genes in Acetobacter diazotrophicus . Soil Biol. Biochem., 29, 871–874. [Google Scholar]
- 89. Meletzus, D. , Teixeira, K. , Perlova, O. , Nawroth, R. et al., 1998. Characterization of genes involved in regulation of nitrogen fixation and ammonium sensing in Acetobacter diazotrophicus, an endophyte of sugarcane, in: Elmerich, C. , Kondorosi, A. , Newton, W.E. (Eds.), Biological Nitrogen Fixatation, 21st Century, vol. 31, Springer, Netherlands, 125–126. [Google Scholar]
- 90. Teixeira, K.R.S. , Wülling, M. , Morgan, T. , Galler, R. et al., 1999. Molecular analysis of the chromosomal region encoding the nifA and nifB genes of Acetobacter diazotrophicus . FEMS Microbiol. Lett., 176, 301–309. [Google Scholar]
- 91. Lee, S. , Reth, A. , Meletzus, D. , Sevilla, M. et al., 2000. Characterization of a major cluster of nif, fix, and associated genes in a sugarcane endophyte, Acetobacter diazotrophicus . J. Bacteriol., 182, 7088–7091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Perlova, O. , Ureta, A. , Nordlund, S. , Meletzus, D. , 2003. Identification of three genes encoding PII‐like proteins in Gluconacetobacter diazotrophicus: studies of their role(s) in the control of nitrogen fixation. J. Bacteriol., 185, 5854–5861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Azuma, Y. , Hosoyama, A. , Matsutani, M. , Furuya, N. et al., 2009. Whole‐genome analyses reveal genetic instability of Acetobacter pasteurianus . Nucleic Acids Res., 37, 5768–5783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Matsutani, M. , Hirakawa, H. , Yakushi, T. , Matsushita, K. , 2011. Genome‐wide phylogenetic analysis of Gluconobacter, Acetobacter, and Gluconacetobacter: Genome‐wide phylogenetic analysis of AAB. FEMS Microbiol. Lett., 315, 122–128. [DOI] [PubMed] [Google Scholar]
- 95. Andres‐Barrao, C. , Falquet, L. , Calderon‐Copete, S.P. , Descombes, P. et al., 2011. Genome sequences of the high‐acetic acid‐resistant bacteria Gluconacetobacter europaeus LMG 18890T and G. europaeus LMG 18494 (Reference Strains), G. europaeus 5P3, and Gluconacetobacter oboediens 174Bp2 (Isolated from Vinegar). J. Bacteriol., 193, 2670–2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Dos Santos, R.A.C. , Berretta, A.A. , Barud, H.D.S. , Ribeiro, S.J.L. et al., 2014. Draft genome sequence of Komagataeibacter rhaeticus strain AF1, a high producer of cellulose, isolated from Kombucha Tea. Genome Announc., 2, e00731‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Illeghems, K. , De Vuyst, L. , Weckx, S. , 2013. Complete genome sequence and comparative analysis of Acetobacter pasteurianus 386B, a strain well‐adapted to the cocoa bean fermentation ecosystem. BMC Genomics, 14, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Chouaia, B. , Gaiarsa, S. , Crotti, E. , Comandatore, F. et al., 2014. Acetic acid bacteria genomes reveal functional traits for adaptation to life in insect guts. Genome Biol. Evol., 6, 912–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Higashiura, N. , Hadano, H. , Hirakawa, H. , Matsutani, M. et al., 2014. Draft genomic DNA sequence of the facultatively methylotrophic bacterium Acidomonas methanolica type strain MB58. FEMS Microbiol. Lett., 351, 9–13. [DOI] [PubMed] [Google Scholar]
- 100. Guedes, H.V. , Rouws, L.F.M. , Oliveira, A.L.M. , Simões‐Araújo, J.L. et al., 2008. Gluconacetobacter diazotrophicus Pal5 strain: selection and characterization of mutants deficient in nitrogen‐fixation ability, in: Dakora, F.D. , Chimphango, S.B.M. , Valentine, A.J. , Elmerich, C. , Newton, W.E. (Eds.), Bioogical Nitrogen Fixation: Towards Poverty Alleviation through Sustainable Agriculture, vol. 42., Springer Netherlands, Dordrecht, 321–322. [Google Scholar]
- 101. Lery, L.M.S. , Coelho, A. , von Kruger, W.M.A. , Gonçalves, M.S.M. et al., 2008. Protein expression profile of Gluconacetobacter diazotrophicus PAL5, a sugarcane endophytic plant growth‐promoting bacterium. Proteomics, 8, 1631–1644. [DOI] [PubMed] [Google Scholar]
- 102. Lery, L.M.S. , von Krüger, W.M.A. , Viana, F.C. , Teixeira, K.R.S. et al., 2008. A comparative proteomic analysis of Gluconacetobacter diazotrophicus PAL5 at exponential and stationary phases of cultures in the presence of high and low levels of inorganic nitrogen compound. Biochim. Biophys. Acta, 1784, 1578–1589. [DOI] [PubMed] [Google Scholar]
- 103. Rouws, L.F.M. , Simões‐Araújo, J.L. , Hemerly, A.S. , Baldani, J.I. , 2008. Validation of a Tn5 transposon mutagenesis system for Gluconacetobacter diazotrophicus through characterization of a flagellar mutant. Arch. Microbiol., 189, 397–405. [DOI] [PubMed] [Google Scholar]
- 104. Dos Santos, M.F. , Muniz de Pádua, V.L. , de Matos Nogueira, E. , Hemerly, A.S. et al., 2010. Proteome of Gluconacetobacter diazotrophicus co‐cultivated with sugarcane plantlets. J. Proteomics, 73, 917–931. [DOI] [PubMed] [Google Scholar]
- 105. Meneses, C.H. , Rouws, L.F. , Simões‐Araújo, J.L. , Vidal, M.S. et al., 2011. Exopolysaccharide production is required for biofilm formation and plant colonization by the nitrogen‐fixing endophyte Gluconacetobacter diazotrophicus . Mol. Plant. Microbe Interact., 24, 1448–1458. [DOI] [PubMed] [Google Scholar]
- 106. Urzúa, L.S. , Vázquez‐Candanedo, A.P. , Sánchez‐Espíndola, A. , Ramírez, C.Á. et al., 2013. Identification and characterization of an iron ABC transporter operon in Gluconacetobacter diazotrophicus Pal 5. Arch. Microbiol., 195, 431–438. [DOI] [PubMed] [Google Scholar]
- 107. Serrato, R.V. , Meneses, C.H.S.G. , Vidal, M.S. , Santana‐Filho, A.P. et al., 2013. Structural studies of an exopolysaccharide produced by Gluconacetobacter diazotrophicus Pal5. Carbohydr. Polym., 98, 1153–1159. [DOI] [PubMed] [Google Scholar]
- 108. Bertini, E.V. , Nieto Peñalver, C.G. , Leguina, A.C. , Irazusta, V.P. et al., 2014. Gluconacetobacter diazotrophicus PAL5 possesses an active quorum sensing regulatory system. Antonie Van Leeuwenhoek, 106, 497–506. [DOI] [PubMed] [Google Scholar]
- 109. Menendez, C. , Banguela, A. , Caballero‐Mellado, J. , Hernandez, L. , 2009. Transcriptional regulation and signal‐peptide‐dependent secretion of exolevanase (LsdB) in the endophyte Gluconacetobacter diazotrophicus . Appl. Environ. Microbiol., 75, 1782–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Alquéres, S.M.C. , Oliveira, J.H.M. , Nogueira, E.M. , Guedes, H.V. et al., 2010. Antioxidant pathways are up‐regulated during biological nitrogen fixation to prevent ROS‐induced nitrogenase inhibition in Gluconacetobacter diazotrophicus . Arch. Microbiol., 192, 835–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Alquéres, S. , Meneses, C. , Rouws, L. , Rothballer, M. et al., 2013. The bacterial superoxide dismutase and glutathione reductase are crucial for endophytic colonization of rice roots by Gluconacetobacter diazotrophicus PAL5. Mol. Plant. Microbe Interact., 26, 937–945. [DOI] [PubMed] [Google Scholar]
- 112. Tejera, N.A. , Ortega, E. , Rodés, R. , Lluch, C. , 2004. Influence of carbon and nitrogen sources on growth, nitrogenase activity, and carbon metabolism of Gluconacetobacter diazotrophicus . Can. J. Microbiol., 50, 745–750. [DOI] [PubMed] [Google Scholar]
- 113. Alquéres, S.M. , Cardoso, A.M. , Brito‐Moreira, J. , Baldani, J.I. et al., 2012. Transfer RNA‐dependent asparagine biosynthesis in Gluconacetobacter diazotrophicus and its influence on biological nitrogen fixation. Plant Soil, 356, 209–216. [Google Scholar]
- 114. Tejera, N. , Ortega, E. , Rodes, R. , Lluch, C. , 2006. Nitrogen compounds in the apoplastic sap of sugarcane stem: some implications in the association with endophytes. J. Plant Physiol., 163, 80–85. [DOI] [PubMed] [Google Scholar]
- 115. Loiret, F.G. , Grimm, B. , Hajirezaei, M.R. , Kleiner, D. et al., 2009. Inoculation of sugarcane with Pantoea sp. increases amino acid contents in shoot tissues; serine, alanine, glutamine and asparagine permit concomitantly ammonium excretion and nitrogenase activity of the bacterium. J. Plant Physiol., 166, 1152–1161. [DOI] [PubMed] [Google Scholar]
- 116. Kerby, R.L. , Roberts, G.P. , 2011. Sustaining N2‐dependent growth in the presence of CO. J. Bacteriol., 193, 774–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Hoffmann, M.‐C. , Pfander, Y. , Fehringer, M. , Narberhaus, F. et al., 2014. NifA‐ and CooA‐coordinated cowN expression sustains nitrogen fixation by Rhodobacter capsulatus in the presence of carbon monoxide. J. Bacteriol., 196, 3494–3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Pedraza, R.O. , 2008. Recent advances in nitrogen‐fixing acetic acid bacteria. Int. J. Food Microbiol., 125, 25–35. [DOI] [PubMed] [Google Scholar]
- 119. Eskin, N. , Vessey, K. , Tian, L. , 2014. Research progress and perspectives of nitrogen fixing bacterium, Gluconacetobacter diazotrophicus, in Monocot plants. Int. J. Agron., 1–13. [Google Scholar]
- 120. James, E.K. , Reis, V.M. , Olivares, F.L. , Baldani, J.I. et al., 1994. Infection of sugar cane by the nitrogen‐fixing bacterium Acetobacter diazotrophicus . J. Exp. Bot., 45, 757–766. [Google Scholar]
- 121. James, E.K. , Olivares, F.L. , 1998. Infection and colonization of sugar cane and other graminaceous plants by endophytic diazotrophs. Crit. Rev. Plant Sci., 17, 77–119. [Google Scholar]
- 122. James, E.K. , Olivares, F.L. , de Oliveira, A.L.M. , dos Reis, F.B. et al., 2001. Further observations on the interaction between sugar cane and Gluconacetobacter diazotrophicus under laboratory and greenhouse conditions. J. Exp. Bot., 52, 747–760. [DOI] [PubMed] [Google Scholar]
- 123. Luna, M.F. , Galar, M.L. , Aprea, J. , Molinari, M.L. et al., 2010. Colonization of sorghum and wheat by seed inoculation with Gluconacetobacter diazotrophicus . Biotechnol. Lett., 32, 1071–1076. [DOI] [PubMed] [Google Scholar]
- 124. Rouws, L.F.M. , Meneses, C.H.S.G. , Guedes, H.V. , Vidal, M.S. et al., 2010. Monitoring the colonization of sugarcane and rice plants by the endophytic diazotrophic bacterium Gluconacetobacter diazotrophicus marked with gfp and gusA reporter genes: Gfp marked G. diazotrophicus on rice. Lett. Appl. Microbiol., 51, 325–330. [DOI] [PubMed] [Google Scholar]
- 125. de Oliveira, A.L.M. , de Canuto, E.L. , Reis, V.M. , Baldani, J.I. , 2003. Response of micropropagated sugarcane varieties to inoculation with endophytic diazotrophic bacteria. Braz. J. Microbiol., 34, 59–61. [Google Scholar]
- 126. Moraes, V.A. , Tauk‐Tornisielo, S.M. , Efeito da inoculação de Acetobacter diazotrophicus em cana‐de‐açúcar (Saccharum spp) variedade SP70‐1143, a partir de cultura de meristemas, in: XIX Congresso Brasileiro de Microbiologia. Rio de Janeiro, BR: 1997. p. 215. [Google Scholar]
- 127. De Oliveira, A.L.M. , de Canuto, E.L. , Urquiaga, S. , Reis, V.M. et al., 2006. Yield of micropropagated sugarcane varieties in different soil types following inoculation with diazotrophic bacteria. Plant Soil, 284, 23–32. [Google Scholar]
- 128. Sevilla, M. , Burris, R.H. , Gunapala, N. , Kennedy, C. , 2001. Comparison of benefit to sugarcane plant growth and 15N2 incorporation following inoculation of sterile plants with Acetobacter diazotrophicus wild‐type and Nif‐ mutant strains. Mol. Plant. Microbe Interact., 14, 358–366. [DOI] [PubMed] [Google Scholar]
- 129. Riggs, P.J. , Chelius, M.K. , Iniguez, A.L. , Kaeppler, S.M. et al., 2001. Enhanced maize productivity by inoculation with diazotrophic bacteria. Funct. Plant Biol., 28, 829–836. [Google Scholar]
- 130. Bastián, F. , Cohen, A. , Piccoli, P. , Luna, V. et al., 1998. Production of indole‐3‐acetic acid and gibberellins A1 and A3 by Acetobacter diazotrophicus and Herbaspirillum seropedicae in chemically‐defined culture media. Plant Growth Regul., 24, 7–11. [Google Scholar]
- 131. Saravanan, V.S. , Madhaiyan, M. , Osborne, J. , Thangaraju, M. et al., 2008. Ecological occurrence of Gluconacetobacter diazotrophicus and nitrogen‐fixing Acetobacteraceae members: their possible role in plant growth promotion. Microb. Ecol., 55, 130–140. [DOI] [PubMed] [Google Scholar]
- 132. Galar, M.L. , Boiardi, J.L. , 1995. Evidence for a membrane‐bound pyrroloquinoline quinone‐linked glucose dehydrogenase in Acetobacter diazotrophicus . Appl. Microbiol. Biotechnol., 43, 713–716. [Google Scholar]
- 133. Maheshkumar, K.S. , Krishnaraj, P.U. , Alagawadi, A.R. , 1999. Mineral phosphate solubilizing activity of Acetobacter diazotrophicus: a bacterium associated with sugar cane. Curr. Sci., 76, 874–875. [Google Scholar]
- 134. Saravanan, V.S. , Kalaiarasan, P. , Madhaiyan, M. , Thangaraju, M. , 2007. Solubilization of insoluble zinc compounds by Gluconacetobacter diazotrophicus and the detrimental action of zinc ion (Zn 2+) and zinc chelates on root knot nematode Meloidogyne incognita . Lett. Appl. Microbiol., 44, 235–241. [DOI] [PubMed] [Google Scholar]
- 135. Intorne, A.C. , Oliveira, M.V.V. , Lima, M.L. , da Silva, J.F. et al., 2009. Identification and characterization of Gluconacetobacter diazotrophicus mutants defective in the solubilization of phosphorus and zinc. Arch. Microbiol., 191, 477–483. [DOI] [PubMed] [Google Scholar]
- 136. Sarathambal, C. , Thangaraju, M. , Paulraj, C. , Gomathy, M. , 2010. Assessing the zinc solubilization ability of Gluconacetobacter diazotrophicus in maize rhizosphere using labelled 65Zn compounds. Indian J. Microbiol., 50, 103–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Crespo, J.M. , Boiardi, J.L. , Luna, M. F. , 2011. Mineral phosphate solubilization activity of Gluconacetobacter diazotrophicus under P‐limitation and plant root environment. Agric. Sci., 02, 16–22. [Google Scholar]
- 138. Natheer, S.E. , Muthukkaruppan, S. , 2012. Assessing the in vitro zinc solubilization potential and improving sugarcane growth by inoculating Gluconacetobacter diazotrophicus . Ann. Microbiol., 62, 435–441. [Google Scholar]
- 139. Saravanan, V.S. , Osborne, J. , Madhaiyan, M. , Mathew, L. et al., 2007. Zinc metal solubilization by Gluconacetobacter diazotrophicus and induction of pleomorphic cells. J. Microbiol. Biotechnol., 17, 1477–1482. [PubMed] [Google Scholar]
- 140. Intorne, A.C. , de Oliveira, M.V.V. , de Pereira, M.L. , de Souza Filho, G.A. , 2012. Essential role of the czc determinant for cadmium, cobalt and zinc resistance in Gluconacetobacter diazotrophicus PAl 5. Int. Microbiol., 15, 69–78. [DOI] [PubMed] [Google Scholar]
- 141. Arencibia, A.D. , Vinagre, F. , Estevez, Y. , Bernal, A. et al., 2006. Gluconacetobacter diazotrophicus elicits a sugarcane defense response against a pathogenic bacteria Xanthomonas albilineans . Plant Signal Behav., 1, 265–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Blanco, Y. , Blanch, M. , Piñón, D. , Legaz, M. et al., 2005. Antagonism of Gluconacetobacter diazotrophicus (a sugarcane endosymbiont) against Xanthomonas albilineans (pathogen) studied in alginate‐immobilized sugarcane stalk tissues. J. Biosci. Bioeng., 99, 366–371. [DOI] [PubMed] [Google Scholar]
- 143. Muthukumarasamy, R. , Revathi, G. , Vadivelu, M. , 2000. Antagonistic potential of N2‐fixing Acetobacter diazotrophicus against Colletotrichum falcatum Went., a causal organism of red‐rot of sugarcane. J. Curr. Sci., 78, 1063–1065. [Google Scholar]
- 144. Mehnaz, S. , Lazarovits, G. , 2006. Inoculation effects of Pseudomonas putida, Gluconacetobacter azotocaptans, and Azospirillum lipoferum on corn plant growth under greenhouse conditions. Microb. Ecol., 51, 326–335. [DOI] [PubMed] [Google Scholar]
- 145. Logeshwaran, P. , Thangaraju, M. , Rajasundari, K. , 2011. In vitro suppression of soil borne pathogenic fungi and pyoluteorin production by Gluconacetobacter diazotrophicus . J. Basic Appl. Sci. Res., 1, 150–156. [Google Scholar]
- 146. de Matos Nogueira, E. , Vinagre, F. , Masuda, H.P. , Vargas, C. et al., 2001. Expression of sugarcane genes induced by inoculation with Gluconacetobacter diazotrophicus and Herbaspirillum rubrisubalbicans. Genet. Mol. Biol., 24, 199–206. [Google Scholar]
- 147. Vargas, C. , De Pádua, V. , Nogueira, E. , Vinagre, F. et al., 2003. Signalling pathways mediating the association between sugarcane and endophytic diazotrophic bacteria: a genomic approach. Symbiosis, 35, 159–180. [Google Scholar]
- 148. Cavalcante, J. , Vargas, C. , Nogueira, E. , Vinagre, F. et al., 2006. Members of the ethylene signalling pathway are regulated in sugarcane during the association with nitrogen‐fixing endophytic bacteria. J. Exp. Bot., 58, 673–686. [DOI] [PubMed] [Google Scholar]
- 149. Chawla, N. , Anand, R. , Narula, N. , 2013. Colonization behaviour of Gluconoacetobacter diazotrophicus in root‐knot nematode (Meloidogyne incognita) infected and healthy cotton plants. Int. J. Microb. Resour. Technol., 2, 24–35. [Google Scholar]
- 150. Yamada, Y. , Yukphan, P. , Lan Vu, H.T. , Muramatsu, Y. et al., 2013. Validation list 149: list of new names and new combinations previously effectively, but not validly, published. Int. J. Syst. Evol. Microbiol., 63, 1–5. [Google Scholar]
- 151. Yukphan, P. , 2004. Asaia krungthepensis sp. nov., an acetic acid bacterium in the α‐Proteobacteria. Int. J. Syst. Evol. Microbiol., 54, 313–316. [DOI] [PubMed] [Google Scholar]
- 152. Malimas, T. , Yukphan, P. , Takahashi, M. , Kaneyasu, M. et al., 2008. Asaia lannaensis sp. nov., a new acetic acid bacterium in the alphaproteobacteria. Biosci. Biotechnol. Biochem., 72, 666–671. [DOI] [PubMed] [Google Scholar]
- 153. Crotti, E. , Rizzi, A. , Chouaia, B. , Ricci, I. et al., 2010. Acetic Acid Bacteria, newly emerging symbionts of insects. Appl. Environ. Microbiol., 76, 6963–6970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Kautz, S. , Rubin, B.E.R. , Moreau, C.S. , 2013. Bacterial infections across the ants: frequency and prevalence of Wolbachia, Spiroplasma, and Asaia . Psyche J. Entomol., 2013, Article ID 936341, 11p. [Google Scholar]
- 155. Jambukar, G.S. , Wange, S.S. , 2005. Field studies on response of sugar beet to microbial inoculants under graded nitrogen levels. J. Soils Crops, 15, 290–296. [Google Scholar]
- 156. Tamura, K. , Peterson, D. , Peterson, N. , Stecher, G. et al., 2011. MEG A5: Molecular Evolutionary Genetics Analysis Using Maximum Likelihood, Evolutionary Distance, and Maximum Parsimony methods. Mol. Biol. Evol., 28, 2731–2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Attwood, M.M. , van Dijken, J.P. , Pronk, J.T. , 1991. Glucose metabolism and gluconic acid production by Acetobacter diazotrophicus . J. Ferment. Bioeng., 72, 101–105. [Google Scholar]
- 158. Vose, P.B. , Ruschel, A.P. , Victoria, R.L. , Matsui, E. , 1981. Potential N2‐fixation by sugarcane, Saccharum sp. in solution culture‐I. Effect of NH4 + vs. NO3− , variety and nitrogen level. In: Associative N2‐Fixation, vol. II, Vose, P.B. , Ruschel, A.P. (Eds.), CRC Press, Boca Raton, p. 119–123. [Google Scholar]
- 159. Fuentes‐Ramirez, L.E. , Jimenez‐Salgado, T. , Abarca‐Ocampo, I.R. , Caballero‐Mellado, J. , 1993. Acetobacter diazotrophicus, an indoleacetic acid producing bacterium isolated from sugarcane cultivars of Mexico. Plant Soil, 154, 145–150. [Google Scholar]
- 160. Muthukumarasamy, R. , Revathi, G. , Lakshminarasimhan, C. , 1999. Influence of N fertilisation on the isolation of Acetobacter diazotrophicus and Herbaspirillum spp. from Indian sugarcane varieties. Biol. Fertil. Soils, 29, 157–164. [Google Scholar]
- 161. Muthukumarasamy, R. , Revathi, G. , Loganathan, P. , 2002. Effect of inorganic N on the population, in vitro colonization and morphology of Acetobacter diazotrophicus (syn. Gluconacetobacter diazotrophicus). Plant Soil, 243, 91–102. [Google Scholar]
- 162. Muñoz‐Rojas, J. , Caballero‐Mellado, J. , 2003. Population dynamics of Gluconacetobacter diazotrophicus in sugarcane cultivars and its effect on plant growth. Microb. Ecol., 46, 454–464. [DOI] [PubMed] [Google Scholar]
- 163. Dos Reis Jr, F.B. , Reis, VM , Urquiaga, S. , Döbereiner, J. , 2000. Influence of nitrogen fertilisation on the population of diazotrophic bacteria Herbaspirillum spp. and Acetobacter diazotrophicus in sugar cane (Saccharum spp.). Plant Soil, 219, 153–159. [Google Scholar]
- 164. Medeiros, A.F.A. , Polidoro, J.C. , Reis, V.M. , 2006. Nitrogen source effect on Gluconacetobacter diazotrophicus colonization of sugarcane (Saccharum spp.). Plant Soil, 279, 141–152. [Google Scholar]
- 165. Suman, A. , Gaur, A. , Shrivastava, A.K. , Yadav, R.L. , 2005. Improving sugarcane growth and nutrient uptake by inoculating Gluconacetobacter diazotrophicus . Plant Growth Regul., 47, 155–162. [Google Scholar]
- 166. Suman, A. , Shrivastava, A.K. , Gaur, A. , Singh, P. et al., 2007. Nitrogen use efficiency of sugarcane in relation to its BNF potential and population of endophytic diazotrophs at different N levels. Plant Growth Regul., 54, 1–11. [Google Scholar]
- 167. Suman, A. , Singh, P. , Lal, M. , 2013. Effects of diverse habitat biofertilizers on yield and nitrogen balance in plant‐ratoon crop cycle of sugarcane in Subtropics. Sugar Tech, 15, 36–43. [Google Scholar]
- 168. De la Cruz, C.P.P. , Bird, C.O. , Isulat, M.D. , 2012. Sprouting, survival and growth of young sugarcane (Saccharum officinarum L.) treated with diazotrophic bacteria (Gluconacetobacter diazotrophicus). Philipp. Agric. Sci., 95, 106–111. [Google Scholar]
- 169. de Oliveira, A. , Urquiaga, S. , Döbereiner, J. , Baldani, J.I. , 2002. The effect of inoculating endophytic N2‐fixing bacteria on micropropagated sugarcane plants. Plant Soil, 242, 205–215. [Google Scholar]
- 170. Schultz, N. , Morais, R.F. , Silva, J.A. , Baptista, R.B. et al., 2012. Avaliação agronômica de variedades de cana‐de‐açúcar inoculadas com bactérias diazotróficas e adubadas com nitrogênio. Pesqui. Agropecuária Bras., 47, 261–268. [Google Scholar]
- 171. Tian, G. , Pauls, P. , Dong, Z. , Reid, L.M. et al., 2009. Colonization of the nitrogen‐fixing bacterium Gluconacetobacter diazotrophicus in a large number of Canadian corn plants. Can. J. Plant Sci., 89, 1009–1016. [Google Scholar]
- 172. Hernandez, L. , Arrieta, J. , Menendez, C. , Vazquez, R. et al., 1995. Isolation and enzymic properties of levansucrase secreted by Acetobacter diazotrophicus SRT4, a bacterium associated with sugar cane. Biochem. J, 309, 113–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Menéndez, C. , Hernández, L. , Banguela, A. , País, J. , 2004. Functional production and secretion of the Gluconacetobacter diazotrophicus fructose‐releasing exo‐levanase (LsdB) in Pichia pastoris. Enzyme Microb. Technol., 34, 446–452. [Google Scholar]
- 174. Muñoz‐Rojas, J. , Fuentes‐Ramírez, L.E. , Caballero‐Mellado, J. , 2005. Antagonism among Gluconacetobacter diazotrophicus strains in culture media and in endophytic association. FEMS Microbiol. Ecol., 54, 57–66. [DOI] [PubMed] [Google Scholar]
- 175. Nita, P. , Pallavi, G. , Shubhangi, S. , Hemlata, S. et al., 2012. Liquid formulations of Acetobacter diazotrophicus L1 and Herbaspirillum seropedicae J24 and their field trials on wheat. Int. J. Environ. Sci., 3, 1116–1129. [Google Scholar]
- 176. Da Silva, M.F. , de Souza Antônio, C. , de Oliveira, P.J. , Xavier, G.R. et al., 2012. Survival of endophytic bacteria in polymer‐based inoculants and efficiency of their application to sugarcane. Plant Soil, 356, 231–243. [Google Scholar]
- 177. Weber, O.B. , Lima, R.N. , Crisóstomo, L.A. , Freitas, J.A.D. et al., 2010. Effect of diazotrophic bacterium inoculation and organic fertilization on yield of Champaka pineapple intercropped with irrigated sapota. Plant Soil, 327, 355–364. [Google Scholar]


