Abstract
The objective of this study was to determine the incidence of respiratory viruses associated with severe pneumonia among children less than 2 years of age in the rural district of Matiari in Sindh, Pakistan. This study was a community‐based prospective cohort active surveillance of infants enrolled at birth and followed for 2 years. Cases were identified using the World Health Organization's Integrated Management of Childhood Illnesses’ definition of severe pneumonia. Nasopharyngeal swabs were obtained for assessment by multiplex RT‐PCR for eight viruses and their subtypes, including RSV, influenza virus, human metapneumovirus, enterovirus/rhinovirus, coronavirus, parainfluenza virus, adenovirus, and human bocavirus. Blood cultures were collected from febrile participants. A total of 817 newborns were enrolled and followed with fortnightly surveillance for 2 years, accounting for a total of 1,501 child‐years of follow‐up. Of the nasopharyngeal swabs collected, 77.8% (179/230) were positive for one or more of the above mentioned respiratory viruses. The incidence of laboratory confirmed viral‐associated pneumonia was 11.9 per 100 child‐years of follow‐up. Enterovirus/rhinovirus was detected in 51.7% patients, followed by parainfluenza virus type III (8.3%), and RSV (5.7%). Of the uncontaminated blood cultures, 1.4% (5/356) were positive. Respiratory viruses are frequently detected during acute respiratory infection episodes in children under 2 years old in a rural community in Pakistan. However, causal association is yet to be established and the concomitant role of bacteria as a co‐infection or super‐infection needs further investigation. J. Med. Virol. 88:1882–1890, 2016 . © 2016 Wiley Periodicals, Inc.
Keywords: multiplex, Pakistan, PCR, pneumonia, viral
Abbreviations
- NP
nasopharyngeal
- ARIs
acute respiratory infections
INTRODUCTION
Acute respiratory infections (ARIs) are among the leading causes of child morbidity and mortality [Liu et al., 2012, 2015; Nair et al., 2013]. In 2011, 1.3 million episodes of pneumonia were fatal in children under 5 years of age [Nair et al., 2013; Walker et al., 2013]. Pakistan is one of the 15 countries in the world that accounts for more than 60% of the worldwide disease burden of pneumonia [Liu et al., 2015; Walker et al., 2013]. Although developing countries such as Pakistan have the greatest burden of pneumonia, national data available on epidemiology and etiology of pneumonia is based on studies published over 2 decades ago [Selwyn, 1990]. Since then, diagnostic modalities and treatments have evolved. Widespread introduction of several vaccines in national immunization programs has reduced and modified the current etiologies of infectious diseases, including etiologies of pneumonia [Measham et al., 2006; Theodoratou et al., 2010]. The modern nucleic acid based molecular diagnostic methods can capture viral respiratory pathogens and with greater specificity and sensitivity than previously possible [Weinberg et al., 2004; Mahony, 2008; Caliendo, 2011].
Common viruses associated with ARIs include influenza A and B viruses, respiratory syncytial virus (RSV), parainfluenza viruses types I–IV, adenovirus, enterovirus, rhinovirus, and human metapneumovirus [Brittain‐Long et al., 2008; Tiveljung‐Lindell et al., 2009]. Since the beginning of this millennia, new viruses have been described including human metapneumovirus, human coronavirus NL63 and HKU1, and human bocavirus [van den Hoogen et al., 2001; Allander et al., 2005; Woo et al., 2005; van der Hoek et al., 2010]. Novel respiratory viruses have the potential to cause pandemics. It is, therefore, important to delineate the burden of viral pneumonia in order to develop new algorithms for healthcare management as well as to consider priorities for future vaccine development.
In this study, we conducted population surveillance over a 33‐month period to determine the incidence of eight viruses and their subtypes, including RSV, influenza virus, human metapneumovirus, enterovirus/rhinovirus, coronavirus, parainfluenza virus, adenovirus, and human bocavirus that were associated with severe pneumonia in children less than 2 years of age in a rural district of Sindh, Pakistan using multiplex PCR assay detection of the selected pathogens.
METHODS
Study Design and Site
The study was a prospective community‐based active surveillance of newborns enrolled at birth and then followed during the period October 2011–June 2014. The study was conducted in the rural district of Matiari, located in Sindh province about 200 km north of Karachi, in Pakistan. Within the district there are 19 union councils, which are the smallest administrative divisions. The selected union councils for this study—Shah Alam Shah Ji Wasi and Sekhat/Khyber—had a population of 60,674 and 12,085 children under 59 months, respectively. The available health facilities in the district include a basic health unit, a rural health center, and a linked referral hospital. The Department of Pediatrics and Child Health at Aga Khan University, Pakistan has established research infrastructure here for community monitoring.
Case Definition
The Integrated Management of Neonatal and Childhood Illnesses (IMCI) World Health Organization (WHO) case definition of severe pneumonia was used in this study. This definition includes the presence of chest indrawing and/or rapid breathing (i.e., children under 2 months of age ≥60 breaths/min; children between 2 and 12 months of age ≥50 breaths/min; children over 12 months–5 years of age ≥40 breaths/min) with or without fever [World Health Organization. Dept. of C and Adolescent, 2005]. Children with WHO defined very severe disease were immediately referred and not included in the study.
Inclusion and Exclusion Criteria
Inclusion criteria for study enrollment included newborns up to 14 days without any major congenital abnormality and whose parents or guardians were willing to provide informed consent. Children were excluded if their families planned to move out of the study area during the 6 months after enrollment.
Clinical Surveillance and Data Collection
Newborns were assessed and enrolled during routine pregnancy surveillance of women of reproductive age (13–49 years) by community health workers. At fortnightly visits, the community health workers screened participants for fever (>38.5°C) and symptoms of ARIs, including cough, difficulty breathing, rapid breathing, chest indrawing, wheezing, runny nose, vomiting, and diarrhea. All of the families were provided contact details for the study physician in case their child experienced any respiratory symptoms at any time throughout the study period. These episodes of ARIs were subsequently recorded by the mobile medical teams. Nasopharyngeal (NP) swabs were collected by trained study physicians from the eligible subjects who demonstrated symptoms of severe pneumonia. While NP swabs were collected at all eligible severe pneumonia episodes, blood samples were collected for culture and bio‐banking from febrile patients only. Treatment and/or referral to a nearby healthcare clinic or hospital was offered to all subjects whether they participated in the study or not. Deaths were recorded by community health workers. Verbal autopsy questionnaires were filled for all deceased children under 2 years of age and reviewed by one independent and trained study physician.
Laboratory Techniques
NP swabs were collected using commercially available Universal Transport Medium (UTM, Diagnostic Hybrids Inc., Athens, OH). After collection, the NP samples were placed in a cooler at 4°C and immediately transported to the Matiari district field site laboratory. At the field site laboratory, the samples were stored at 4°C in a designated laboratory refrigerator. The samples were then transported to the Infectious Diseases Research Laboratory (IDRL) at the Aga Khan University in Karachi, using a cold chain protocol with the temperature of the ice chest maintained at 2–8°C with documentation of the temperature at the beginning and the end of the transport process. At IDRL, the samples were logged and recorded using a barcode and participant identification number and then stored in −70°C freezers until further processing for assay testing, allowing only one freeze‐thaw event per sample for an assay. Frozen samples were thawed and spiked with MS2 bacteriophage (extrinsic control) before total nucleic extraction to check the efficiency of nucleic acid amplification. Semi‐automated nucleic acid extraction was performed using a NucliSENS® miniMAG® instrument (bioMérieux, Craponne, France). An xTAG® Respiratory Viral Panel Fast was used for the detection of eight viruses and their subtypes using microsphere‐based Luminex detection technology (Luminex 200 Molecular Diagnostic Inc., Toronto, Canada). Samples were analyzed using TDAS software. Viruses detected by the xTAG® Respiratory Viral Panel Fast included influenza A (non‐specific influenza A, H1, H3, H1N1), influenza B, RSV, parainfluenza (types I–IV), human metapneumovirus, adenovirus, enterovirus/rhinovirus, coronavirus (NL63, HKU1, 229E, OC43), and human bocavirus.
The blood culture samples were collected aseptically at the field site and transported in a Coleman cooler within 2 hr at ambient temperature to the field site lab. The blood culture bottles were placed in a Bactec BD 9050 Instrument programmed for 7 days at 37°C incubation. Any positive signal bottles were removed from the Bactec BD 9050 instrument when an alarm signaled that a bottle was positive for turbidity. Gram stain was performed on the blood culture sample, and the blood sample was cultured onto 5% sheep blood agar (SBA), chocolate agar, and MacConkey agar plates. The SBA and chocolate agar plates were incubated at 5% CO2 in 37°C incubators. The MacConkey agar plates were incubated at 37°C for 24–48 hr. All positive Bactec bottles and all agar plates were sent to AKU IDRL Lab for further identification of the isolate and antibiotic susceptibility testing.
Data Entry and Management
Surveillance data were collected using standardized forms with questions about clinical symptoms over the past 14 days. The data forms were taken back to the field site office, and all data were entered into the computer twice by separate data operators at different computers for the purposes of accuracy and quality control assessment. Data quality was tested by performing error checks simultaneously with dual data entry. Discrepancies were resolved by the field site data manager and again when compiling and uploading data to the database. Corrections or clarifications were documented in a new field while keeping the original data set intact, including detailed rationale for any changes. Visual Foxpro 9.0 was used for designing the database, the data entry software, and the procedures for data quality assurance. Laboratory data were entered into a standardized database that included the date of sample collection, and the participant identification number. All data forms were stored in locked cabinets in a locked room at the field site office or subsequently at the Aga Khan University Pediatric Research Office. Special arrangements were made to enforce referential integrity of the database. SPSS 15.0 was used for data analyses.
Ethical Statement
The study was approved by the Aga Khan University Ethical Review Committee and by the University of Virginia Human Investigation Committee and Institutional Review Board. Written informed consent was obtained from all parents and/or guardians of the participating children. All names and other personal identifying data were removed from the data before uploading to the local database.
RESULTS
Demographics and Symptomatology
During the period of October 2011–June 2014, a total of 817 study participants were enrolled, of which 692 participants were followed throughout the study period (Fig. 1). The number of child‐years of follow‐up was 1,501 (Table I). A total of 409 (50.1%) of the patients were male.
Figure 1.
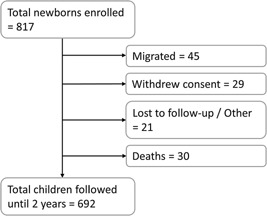
Schematic representation of the study participants enrolled and followed in a rural community in Pakistan during the period of October 2011–June 2014.
Table I.
Viruses and Bacteria Detected in Patients of Severe Pneumonia Aged 0–24 Months in a Rural Community in Pakistan From October 2011 to June 2014
| n (%) | |||
|---|---|---|---|
| Age group | All cases | 0–5 months | 6–23 months |
| Total patients followed | 817 | 817 | 797 |
| Gender | |||
| Male | 409 (50.1) | 409 (50.1) | 399 (50.1) |
| Female | 408 (49.9) | 408 (49.9) | 398 (49.9) |
| Total child‐years of follow‐up | 1,501 | 399 | 1,495 |
| Blood cultures—total number performed | 389 | 212 | 177 |
| Blood cultures performed (excluding contaminants) | 356 | 194 | 162 |
| Positive blood cultures (excluding contaminants) | 5 (1.4) | 4 (2.6) | 1 (0.6) |
| Β‐hemolytic Streptococcus (Group A Streptococcus) | 1 | 1 | 0 |
| Campylobacter | 2 | 2 | 0 |
| Streptococcus pneumoniae | 2 | 1 | 1 |
| Luminex Assays—total number performed | 230 | 201 | 29 |
| Luminex Assays—number of positive assay results | 179 (77.8) | 154 (76.6) | 25 (86.2) |
| Adenovirus | 8 (3.5) | 6 (3) | 2 (6.9) |
| Human bocavirus | 1 (0.4) | 1 (0.5) | 0 (0) |
| Human coronavirus 229 E | 1 (0.4) | 1 (0.5) | 0 (0) |
| Human coronavirus HKU1 | 5 (2.2) | 5 (2.5) | 0 (0) |
| Human coronavirus NL63 | 4 (1.7) | 3 (1.5) | 1 (3.4) |
| Human coronavirus OC43 | 11 (4.8) | 9 (4.5) | 2 (6.9) |
| Enterovirus/rhinovirus | 119 (51.7) | 110 (54.7) | 9 (31) |
| Human metapneumovirus | 5 (2.2) | 1 (0.5) | 4 (13.8) |
| Influenza‐B | 4 (1.7) | 3 (1.5) | 1 (3.4) |
| Parainfluenza virus I | 2 (0.9) | 1 (0.5) | 1 (3.4) |
| Parainfluenza virus II | 2 (0.9) | 1 (0.5) | 1 (3.4) |
| Parainfluenza virus III | 19 (8.3) | 17 (8.5) | 2 (6.9) |
| Parainfluenza virus IV | 10 (4.3) | 10 (5) | 0 (0) |
| RSV | 13 (5.7) | 5 (2.5) | 8 (27.6) |
| Human coronavirus OC43/enterovirus/rhinovirus | 4 (1.7) | 4 (2) | 0 (0) |
| Parainfluenza virus III/enterovirus/rhinovirus | 3 (1.3) | 3 (1.5) | 0 (0) |
| Parainfluenza virus IV/enterovirus/rhinovirus | 3 (1.3) | 3 (1.5) | 0 (0) |
| RSV/enterovirus/rhinovirus | 3 (1.3) | 2 (1) | 1 (3.4) |
| Enterovirus/rhinovirus/adenovirus | 2 (0.9) | 1 (0.5) | 1 (3.4) |
| Human coronavirus 229 E/enterovirus/rhinovirus | 1 (0.4) | 1 (0.5) | 0 (0) |
| Human coronavirus 229 E HKU1/parainfluenza virus III/enterovirus/rhinovirus | 1 (0.4) | 1 (0.5) | 0 (0) |
| Human coronavirus NL63/enterovirus/rhinovirus/human metapneumovirus | 1 (0.4) | 0 (0) | 1 (3.4) |
| Human coronavirus OC43/adenovirus | 1 (0.4) | 1 (0.5) | 0 (0) |
| Human coronavirus OC43/parainfluenza virus III | 1 (0.4) | 1 (0.5) | 0 (0) |
| Enterovirus/rhinovirus/human metapneumovirus | 1 (0.4) | 0 (0) | 1 (3.4) |
| Influenza‐B/RSV | 1 (0.4) | 0 (0) | 1 (3.4) |
| Parainfluenza virus II/enterovirus/rhinovirus | 1 (0.4) | 1 (0.5) | 0 (0) |
| Parainfluenza virus IV/adenovirus | 1 (0.4) | 1 (0.5) | 0 (0) |
| RSV/parainfluenza virus IV | 1 (0.4) | 0 (0) | 1 (3.4) |
| Negative | 51 (22.2) | 47 (23.4) | 4 (13.8) |
Incidence
The overall incidence of severe pneumonia in this cohort was 15.3 (13.5–17.1) episodes per 100 child‐years of follow‐up (Table II). The severe pneumonia incidence rate, based on laboratory confirmed detection of a pathogen, was 11.9 (10.3–13.6) episodes per 100 child‐years. Incidence rates of severe pneumonia per 100 person years associated with individual pathogens were also calculated (Table II). The pathogens with the highest detection rate were enterovirus/rhinovirus at 7.9 (6.6–9.3) episodes per 100 child‐years followed by parainfluenza virus type III at 1.3 (0.7–1.8) episodes per 100 child‐years, and RSV at 0.9 (0.4–1.3) episodes per 100 child‐years.
Table II.
Incidence Rates for Pathogen Associated Severe Pneumonia Among Children Aged 0–24 Months in a Rural Community in Pakistan From October 2011 to June 2014 a
| All cases | 0–5 months | 6–23 months | |
|---|---|---|---|
| Total child years followed | 1,501 | 399 | 1,495 |
| Incidence of severe pneumonia per 100 child year of follow‐up (95%CI) | 15.32 (13.5–17.15) | 50.38 (45.47–55.28) | 1.94 (1.24–2.64) |
| Incidence of viral‐associated severe pneumonia per 100 child year of follow‐up (95%CI) | 11.93 (10.29–13.56) | 38.6 (33.82–43.37) | 1.67 (1.02–2.32) |
| Incidence of Enterovirus/Rhinovirus associated pneumonia per 100 child year of follow‐up (95%CI) | 7.93 (6.56–9.29) | 27.57 (23.18–31.95) | 0.6 (0.21–0.99) |
| Parainfluenza virus III | 1.27 (0.7–1.83) | 4.26 (2.28–6.24) | 0.13 (0.0–0.32) |
| RSV | 0.87 (0.4–1.33) | 1.25 (0.16–2.34) | 0.54 (0.17–0.9) |
| Human coronavirus OC43 | 0.73 (0.3–1.16) | 2.26 (0.8–3.71) | 0.13 (0.0–0.32) |
| Parainfluenza virus IV | 0.67 (0.25–1.08) | 2.51 (0.97–4.04) | — |
| Human metapneumovirus | 0.33 (0.04–0.62) | 0.25 (0.0–0.74) | 0.27 (0.01–0.53) |
| Influenza‐B | 0.27 (0.01–0.53) | 0.75 (0.0–1.6) | 0.07 (0.0–0.2) |
| All viruses | 13.59 (11.86–15.32) | 43.36 (38.5–48.22) | 2.07 (1.35–2.8) |
Association does not mean causality.
Mortalities
There were 30 deaths in total. Nine deaths were due to respiratory causes. Of these, eight were attributable to pneumonia/ARI, and one to measles. Other major causes of death were sepsis (five), meningitis (three), diarrheal diseases (three), severe malnutrition (two), and congenital malformations (two). Causes of death identified in singular cases included tuberculosis, other unspecified non communicable disease and other perinatal cause of death. In three cases, it was not possible to ascertain cause of death from verbal autopsy forms. There were deaths in children during the first month of life, which included a 14 days old baby with pneumonia and 12 days old baby with other specific perinatal cause of death.
Virological Assay Results
Of the pathogens included in the xTAG® Respiratory Viral Panel assay, a total of eight viruses and their subtypes were detected during the course of this study. A total of 230 multiplex PCR assays were performed using the NP swab specimens. Of these samples tested, 77.8% (n = 179) were positive for at least one pathogen, and 10.9% (n = 25) were positive for more than one pathogen (Table I). The majority of NP swab samples collected and analyzed in this study were obtained from children less than 6 months old. Correspondingly, the majority of positive assay results for pneumonia‐associated pathogens were detected in the NP swabs in children under 6 months of age (76.6%; n = 154).
The most frequently detected viruses were enterovirus/rhinovirus at 51.7% (n = 105) followed by parainfluenza virus type III at 8.3% (n = 19), and RSV 5.7% (n = 13) (Fig. 2).
Figure 2.
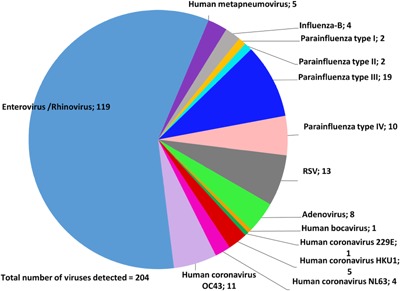
Pie chart displaying the frequency of viruses detected on NP swabs in patients with pneumonia aged 0–24 months in a rural community in Pakistan during the period of October 2011–June 2014. Two or more co‐infecting viruses were counted in their own categories.
Multiple detections of parainfluenza virus type IV (n = 10; 5%) and human coronavirus CoV HKU1 (n = 5; 2.5%) were detected only in children less than 6 months old. Remaining viral pathogens were found in both children less than 6 months old and older than 6 months, or alternatively, detected just once.
Co‐Infections
Of the 10.9% (n = 25) NP swabs that were positive for more than one pathogen, 92% (n = 23) had two pathogens detected, and 8% (n = 2) had more than two pathogens detected. Enterovirus/rhinovirus was detected in combination with other pathogens in 19 cases with the other pathogens being RSV, parainfluenza type IV, corona viruses, adenovirus, and human metapneumovirus. Parainfluenza virus type IV and RSV were each found in combination in five cases. The most common combination was enterovirus/rhinovirus with coronavirus OC43.
Bacterial Culture Results
A total of 389 blood cultures were collected from febrile participants during the course of this study of which a total of 38 were positive for growth. Of these blood cultures, 33 were assessed to represent growth of a contaminant (based on growth of a bacterium that is part of normal skin flora and highly unlikely to be a pathogen) or a false positive (positive alarm on the Bactec sensor with no evidence of any organism on Gram stain or growth on agar plates). Of the 356 remaining uncontaminated blood cultures, 5 (1.4%) were positive (Table I). Streptococcus pneumoniae was detected in two samples. Two participants with Gram stain or culture positive for Campylobacter spp. also had enterovirus/rhinovirus detected on NPs (data not shown).
Seasonality
The average temperature recorded in the study period was 37.5°C (99.5°F). Pneumonia episodes were recorded throughout the first year with a peak of activity noted in April 2012 and a nadir following April 2013 (Fig. 3). ARI case detections became negligible for the remainder of the study period until June 2014 with only one case of ARI detected in September 2013. With the exception of November 2012, enterovirus/rhinovirus was noted to have activity throughout the study period through March 2013; the highest detection rate of enterovirus/rhinovirus in April 2012 overlapped with the highest number of pneumonia episodes detected. Parainfluenza type III was detected between December 2011 and May 2012 and subsequently, one case was detected in September 2013. RSV was detected between November 2011 and February 2012, October 2012 and April 2013. All cases of human metapneumovirus were detected between August 2012 and December 2012.
Figure 3.
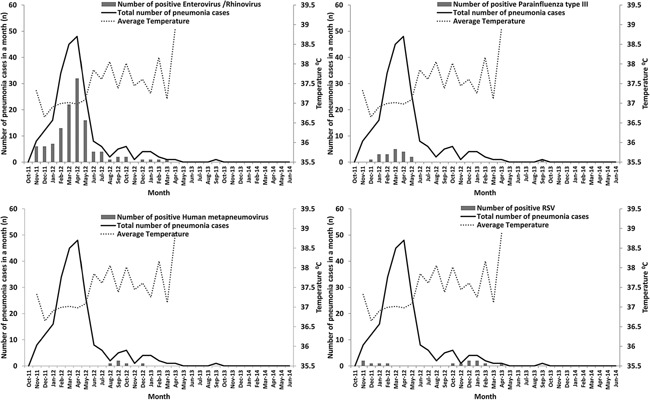
Seasonal distribution of viral pathogens associated with pneumonia in patients 0–24 months in a rural community in Pakistan from October 2011 to June 2014.
DISCUSSION
This study represents an important advance in understanding the role of viruses and bacteria in ARI episodes in children in a rural community setting in Pakistan. It is the first study in Pakistan to utilize molecular diagnostic tests to prospectively determine the burden and seasonality of multiple respiratory viruses including coronaviruses, enterovirus/rhinovirus, and human bocavirus in community setting. In this study population, the incidence of laboratory confirmed viral‐associated severe pneumonia in children <2 years old was 11.9 episodes per 100 child‐years, which was considerably lower than the detection rate reported in a study conducted on inpatient and outpatient wards of children less than 5 years old in rural Kenya (56 cases per 100 child‐years) with active community surveillance [Feikin et al., 2012]. However, the number of pneumonia episodes recorded in our study was higher than that reported from a hospital‐based study with active population surveillance conducted in rural Bangladesh (50.5 hospitalizations per 100,000 patient‐weeks) [Nasreen et al., 2014]. Comparisons are limited due to differences in methodologies and populations being studied.
Within this age group, children less than 6 months old had the highest incidence of viral‐associated pneumonia. Therefore, the highest number of NP swabs was collected from participants in this age group and the highest number of positive samples was detected in this age group. As the age of the cohort increased, the incidence of viral‐associated ARIs declined. This finding corresponds to a birth cohort study conducted in periurban South Africa that also found that children younger than 6 months old are most vulnerable to pneumonia [le Roux et al., 2015].
In this study, 77.8% (n = 179) of NP swab samples were positive for at least one pathogen. The most prevalent viruses detected were enterovirus/rhinovirus, followed by parainfluenza virus type III, and RSV. RSV and parainfluenza virus type III were less prevalent in this study than those conducted in other regions of the world, such as in hospital‐based studies in rural Madagascar and China and in urban slums of Brazil [Weigl et al., 2007; Bezerra et al., 2011; Feikin et al., 2012; Hoffmann et al., 2012; Zhang et al., 2013]. The NP specimen positivity rate for at least one pathogen in this study was higher or at par compared to other countries in the region. For example, a tertiary hospital‐based study of children under 14 years of age in India reported 45.7% NP swab positivity rate [Singh et al., 2014]. The aforementioned study in Bangladesh reported NP swab positivity rates of 52% in hospitalized patients and 55% in non‐hospitalized patients, while a previous study following a birth cohort of children for 2 years reported that 77% of pneumonia episodes were associate with a respiratory virus [Homaira et al., 2012; Nasreen et al., 2014]. RSV and human metapneumovirus had higher rates of detection from NP samples of hospitalized children (6 weeks–2 years old) in an urban setting in Pakistan [Ali et al., 2013].
Endemicity of enterovirus/rhinovirus throughout the year in this study is consistent with the findings of others [Bezerra et al., 2011; Do et al., 2011; Zhang et al., 2013]. In previous literature reports, RSV has been shown to have seasonal variation, occurring usually in either the cold season or the rainy season in different countries [Weber et al., 1998; Bezerra et al., 2011; Hoffmann et al., 2012; Bigogo et al., 2013; Stockman et al., 2013; Zhang et al., 2013]. In this study, RSV also exhibited seasonal variability with a clear pattern of being detected in the winter months in 2 consecutive years, as corroborated by previous studies from within Pakistan as well as neighboring countries [Ghafoor et al., 1990; Zhang et al., 2013; Singh et al., 2014].
Blood culture positivity (1.4%) was low but comparable to aforementioned studies in Kenya (3.3%) and Bangladesh (6%) [Feikin et al., 2012; Nasreen et al., 2014]. This low positivity rate may possibly be due to prior antibiotic use. There were just two cultures positive for S. pneumoniae. Although Pakistan introduced pneumococcal conjugate vaccine (PCV 10) in October 2012 in its Expanded Programme for Immunization, the limited rollout at that time was unlikely to affect subsequent bacterial detection in this study; the overall impact of the limited rollout of the pneumococcal vaccine in Pakistan is still under investigation [GAVI, 2012; Ali, 2014].
The strengths of this study lie in it being the first prospective cohort study to determine the incidence of severe pneumonia that is associated with respiratory viruses in children younger than 2 years old using cutting edge techniques such as multiplex PCR in Pakistan. This study was a community‐based surveillance conducted in a rural area of Pakistan, as opposed to most other studies of pneumonia etiology that have been hospital‐based and/or conducted in urban settings. This study helps bridge the gap since the 1980s’ Board of Science and Technology for International Development (BOSTID) studies determining the epidemiology of ARIs, which included surveillance in Pakistan albeit in a different region [Selwyn, 1990]. Limitations of this study include its observational cohort design as opposed to a case‐control study, which makes it difficult to determine whether these viruses are truly responsible for the symptoms, or whether they are detectable in healthy children as well in that area. Viral detection does not necessarily have a causal association with acute illness. Presence of respiratory viruses in controls has been reported in many other similar studies in the literature, and lack of viral testing from asymptomatic children in our study is a limitation [Mathisen et al., 2010; Singleton et al., 2010; Feikin et al., 2012; Hammitt et al., 2012]. The Luminex assay used in this study, similar to most other commercial assays was unable to distinguish between enterovirus and rhinovirus, hence, the results were grouped together as enterovirus/rhinovirus.
CONCLUSIONS
We found that the incidence of severe pneumonia is particularly high in children younger than 6 months old in our rural study setting of Sindh, with a sharp decline in cases in children older than 6 months old. Respiratory viruses are frequently detected at the time of these pneumonia episodes, though causal association cannot be established based on this study. The role of bacterial co‐infections in these cases cannot be completely ruled out. Nonetheless, it appears that prevention of viruses whether through vaccines or other measures is likely to reduce the burden of pneumonia irrespective of the fact whether these viruses cause the infection alone or as an inciting event for a secondary infection to occur.
ACKNOWLEDGMENTS
We thank the children and their families in Matiari for their participation in this study.
Conflicts of interest: none.
REFERENCES
- Ali A, Khowaja AR, Bashir MZ, Aziz F, Mustafa S, Zaidi A. 2013. Role of human metapneumovirus, influenza a virus and respiratory syncytial virus in causing WHO‐defined severe pneumonia in children in a developing country. PLoS ONE 8:e74756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali SA. 2014. Pre‐ISPPD9 (International Symposium of Pneumococci and Pneumococcal Disease) South Asia Symposium; Pneumoccoal Conjugate Vaccine Impact in Pakistan, Anita Zaidi and Asad Ali, Pneumococcus Research Team, Dept. of Pediatrics and Child Health, Aga Khan University; Date Accessed: 16th March, 2015; http://www.jhsph.edu/research/centers-and-institutes/ivac/resources/ISPPD9-SouthAsiaSymposium/Asad_Ali_Pakistan.pdf. March 9–13, 2014 Hyderabad, India.
- Allander T, Tammi MT, Eriksson M, Bjerkner A, Tiveljung‐Lindell A, Andersson B. 2005. Cloning of a human parvovirus by molecular screening of respiratory tract samples. Proc Natl Acad Sci USA 102:12891–12896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezerra PG, Britto MC, Correia JB, Duarte Mdo C, Fonceca AM, Rose K, Hopkins MJ, Cuevas LE, McNamara PS. 2011. Viral and atypical bacterial detection in acute respiratory infection in children under five years. PLoS ONE 6:e18928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigogo GM, Breiman RF, Feikin DR, Audi AO, Aura B, Cosmas L, Njenga MK, Fields BS, Omballa V, Njuguna H, Ochieng PM, Mogeni DO, Aol GO, Olack B, Katz MA, Montgomery JM, Burton DC. 2013. Epidemiology of respiratory syncytial virus infection in rural and urban Kenya. J Infect Dis 208:S207–S216. [DOI] [PubMed] [Google Scholar]
- Brittain‐Long R, Nord S, Olofsson S, Westin J, Anderson LM, Lindh M. 2008. Multiplex real‐time PCR for detection of respiratory tract infections. J Clin Virol 41:53–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caliendo AM. 2011. Multiplex PCR and emerging technologies for the detection of respiratory pathogens. Clin Infect Dis 52:S326–S330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do AH, van Doorn HR, Nghiem MN, Bryant JE, Hoang TH, Do QH, Van TL Tran TT, Wills B, Nguyen VC, Vo MH, Vo CK, Nguyen MD, Farrar J, Tran TH, de Jong MD. 2011. Viral etiologies of acute respiratory infections among hospitalized Vietnamese children in Ho Chi Minh City, 2004–2008. PLoS ONE 6:e18176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feikin DR, Njenga MK, Bigogo G, Aura B, Aol G, Audi A, Jagero G, Muluare PO, Gikunju S, Nderitu L, Winchell JM, Schneider E, Erdman DD, Oberste MS, Katz MA, Breiman RF. 2012. Viral and bacterial causes of severe acute respiratory illness among children aged less than 5 years in a high malaria prevalence area of western Kenya, 2007–2010. Pediatr Infect Dis J 32:e14–e19. [DOI] [PubMed] [Google Scholar]
- GAVI. 2012. Pakistan is first South Asian country to launch vaccine against childhood pneumonia; October 9th 2012.
- Ghafoor A, Nomani NK, Ishaq Z, Zaidi SZ, Anwar F, Burney MI, Qureshi AW, Ahmad SA. 1990. Diagnoses of acute lower respiratory tract infections in children in Rawalpindi and Islamabad, Pakistan. Rev Infect Dis 12:S907–S914. [DOI] [PubMed] [Google Scholar]
- Hammitt LL, Kazungu S, Morpeth SC, Gibson DG, Mvera B, Brent AJ, Mwarumba S, Onyango CO, Bett A, Akech DO, Murdoch DR, Nokes DJ, Scott JA. 2012. A preliminary study of pneumonia etiology among hospitalized children in Kenya. Clin Infect Dis 54:S190–S199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann J, Rabezanahary H, Randriamarotia M, Ratsimbasoa A, Najjar J, Vernet G, Contamin B, Paranhos‐Baccala G. 2012. Viral and atypical bacterial etiology of acute respiratory infections in children under 5 years old living in a rural tropical area of Madagascar. PLoS ONE 7:e43666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homaira N, Luby SP, Petri WA, Vainionpaa R, Rahman M, Hossain K, Snider CB, Rahman M, Alamgir AS, Zesmin F, Alam M, Gurley ES, Zaman RU, Azim T, Erdman DD, Fry AM, Bresee J, Widdowson MA, Haque R, Azziz‐Baumgartner E. 2012. Incidence of respiratory virus‐associated pneumonia in urban poor young children of Dhaka, Bangladesh, 2009–2011. PLoS ONE 7:e32056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- le Roux DM, Myer L, Nicol MP, Zar HJ. 2015. Incidence and severity of childhood pneumonia in the first year of life in a South African birth cohort: The Drakenstein Child Health Study. Lancet Glob Health 3:e95–e103. [DOI] [PubMed] [Google Scholar]
- Liu L, Johnson HL, Cousens S, Perin J, Scott S, Lawn JE, Rudan I, Campbell H, Cibulskis R, Li M, Mathers C, Black RE. 2012. Global, regional, and national causes of child mortality: An updated systematic analysis for 2010 with time trends since 2000. Lancet 379:2151–2161. [DOI] [PubMed] [Google Scholar]
- Liu L, Oza S, Hogan D, Perin J, Rudan I, Lawn JE, Cousens S, Mathers C, Black RE. 2015. Global, regional, and national causes of child mortality in 2000–13, with projections to inform post‐2015 priorities: An updated systematic analysis. Lancet 385:430–440. [DOI] [PubMed] [Google Scholar]
- Mahony JB. 2008. Detection of respiratory viruses by molecular methods. Clin Microbiol Rev 21:716–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathisen M, Strand TA, Valentiner‐Branth P, Chandyo RK, Basnet S, Sharma BN, Adhikari RK, Hvidsten D, Shrestha PS, Sommerfelt H. 2010. Respiratory viruses in nepalese children with and without pneumonia: A case‐control study. Pediatr Infect Dis J 29:731–735. [DOI] [PubMed] [Google Scholar]
- Measham AR, Alleyne G, Mills A, Musgrove P, Claeson M, Jamison DT, Evans DB, Breman JG, Jha P. 2006. Chapter 25: Acute respiratory tract infections in children. Disease control priorities in developing countries. Washington: DC World Bank and Oxford University Press. [PubMed] [Google Scholar]
- Nair H, Simoes EA, Rudan I, Gessner BD, Azziz‐Baumgartner E, Zhang JS, Feikin DR, Mackenzie GA, Moisi JC, Roca A, Baggett HC, Zaman SM, Singleton RJ, Lucero MG, Chandran A, Gentile A, Cohen C, Krishnan A, Bhutta ZA, Arguedas A, Clara AW, Andrade AL, Ope M, Ruvinsky RO, Hortal M, McCracken JP, Madhi SA, Bruce N, Qazi SA, Morris SS, El Arifeen S, Weber MW, Scott JA, Brooks WA, Breiman RF, Campbell H. 2013. Global and regional burden of hospital admissions for severe acute lower respiratory infections in young children in 2010: A systematic analysis. Lancet 381:1380–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasreen S, Luby SP, Brooks WA, Homaira N, Al Mamun A, Bhuiyan MU, Rahman M, Ahmed D, Abedin J, Rahman M, Alamgir AS, Fry AM, Streatfield PK, Rahman A, Bresee J, Widdowson MA, Azziz‐Baumgartner E. 2014. Population‐based incidence of severe acute respiratory virus infections among children aged <5 years in rural Bangladesh, June–October 2010. PLoS ONE 9:e89978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selwyn BJ. 1990. The epidemiology of acute respiratory tract infection in young children: Comparison of findings from several developing countries. Coordinated Data Group of BOSTID Researchers. Rev Infect Dis 12:S870–S888. [DOI] [PubMed] [Google Scholar]
- Singh AK, Jain A, Jain B, Singh KP, Dangi T, Mohan M, Dwivedi M, Kumar R, Kushwaha RA, Singh JV, Mishra AC, Chhaddha MS. 2014. Viral aetiology of acute lower respiratory tract illness in hospitalised paediatric patients of a tertiary hospital: One year prospective study. Indian J Med Microbiol 32:13–18. [DOI] [PubMed] [Google Scholar]
- Singleton RJ, Bulkow LR, Miernyk K, DeByle C, Pruitt L, Hummel KB, Bruden D, Englund JA, Anderson LJ, Lucher L, Holman RC, Hennessy TW. 2010. Viral respiratory infections in hospitalized and community control children in Alaska. J Med Virol 82:1282–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockman LJ, Brooks WA, Streatfield PK, Rahman M, Goswami D, Nahar K, Rahman MZ, Luby SP, Anderson LJ. 2013. Challenges to evaluating respiratory syncytial virus mortality in Bangladesh, 2004–2008. PLoS ONE 8:e53857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodoratou E, Johnson S, Jhass A, Madhi SA, Clark A, Boschi‐Pinto C, Bhopal S, Rudan I, Campbell H. 2010. The effect of Haemophilus influenzae type b and pneumococcal conjugate vaccines on childhood pneumonia incidence, severe morbidity and mortality. Int J Epidemiol 39:i172–i185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiveljung‐Lindell A, Rotzen‐Ostlund M, Gupta S, Ullstrand R, Grillner L, Zweygberg‐Wirgart B, Allander T. 2009. Development and implementation of a molecular diagnostic platform for daily rapid detection of 15 respiratory viruses. J Med Virol 81:167–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Hoogen BG, de Jong JC, Groen J, Kuiken T, de Groot R, Fouchier RA, Osterhaus AD. 2001. A newly discovered human pneumovirus isolated from young children with respiratory tract disease. Nat Med 7:719–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Hoek L, Ihorst G, Sure K, Vabret A, Dijkman R, de Vries M, Forster J, Berkhout B, Uberla K. 2010. Burden of disease due to human coronavirus NL63 infections and periodicity of infection. J Clin Virol 48:104–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker CL, Rudan I, Liu L, Nair H, Theodoratou E, Bhutta ZA, O'Brien KL, Campbell H, Black RE. 2013. Global burden of childhood pneumonia and diarrhoea. Lancet 381:1405–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber MW, Mulholland EK, Greenwood BM. 1998. Respiratory syncytial virus infection in tropical and developing countries. Trop Med Int Health 3:268–280. [DOI] [PubMed] [Google Scholar]
- Weigl JA, Puppe W, Meyer CU, Berner R, Forster J, Schmitt HJ, Zepp F. 2007. Ten years’ experience with year‐round active surveillance of up to 19 respiratory pathogens in children. Eur J Pediatr 166:957–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg GA, Erdman DD, Edwards KM, Hall CB, Walker FJ, Griffin MR, Schwartz B, New Vaccine Surveillance Network Study G. 2004. Superiority of reverse‐transcription polymerase chain reaction to conventional viral culture in the diagnosis of acute respiratory tract infections in children. J Infect Dis 189:706–710. [DOI] [PubMed] [Google Scholar]
- Woo PC, Lau SK, Chu CM, Chan KH, Tsoi HW, Huang Y, Wong BH, Poon RW, Cai JJ, Luk WK, Poon LL, Wong SS, Guan Y, Peiris JS, Yuen KY. 2005. Characterization and complete genome sequence of a novel coronavirus, coronavirus HKU1, from patients with pneumonia. J Virol 79:884–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. Dept. of C, Adolescent H. 2005. Handbook IMCI: Integrated management of childhood illness. Switzerland: World Health Organization. [Google Scholar]
- Zhang C, Zhu N, Xie Z, Lu R, He B, Liu C, Ma X, Tan W. 2013. Viral etiology and clinical profiles of children with severe acute respiratory infections in china. PLoS ONE 8:e72606. [DOI] [PMC free article] [PubMed] [Google Scholar]


