Figure 5.
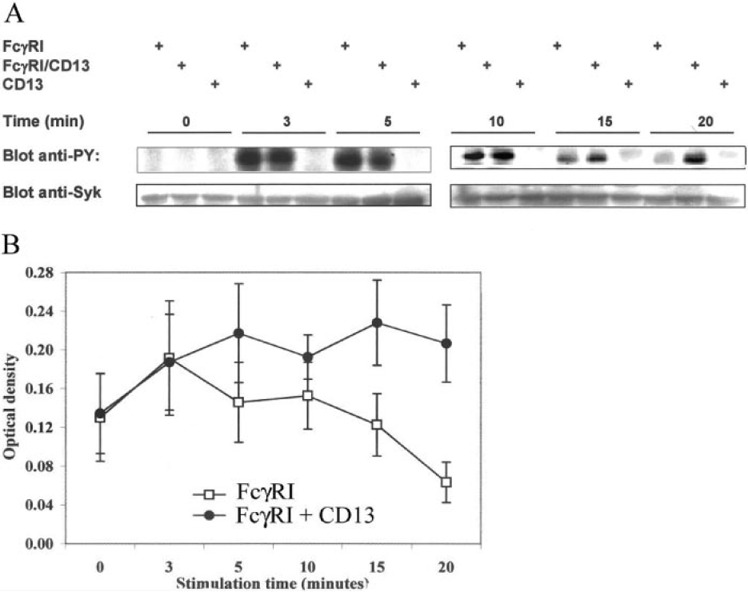
Coaggregation of FcγRI with CD13 induces a prolongation in the Syk phosphorylation level. (A) U‐937 cells were incubated with 10 μg each of anti‐FcγRI mAb, anti‐CD13 mAb, or the combination of both antibodies. After cross‐linking for 3, 5, 10, 15, or 20 min with F(ab)′2 fragments of anti‐mouse antibodies at 37°C, cells were lysed, and proteins were resolved on SDS‐PAGE. Tyrosine‐phosphorylated proteins were blotted with PY20‐PY99 antiphosphotyrosine antibodies (Blot anti‐PY). Bands in the upper panels show the phosphorylated protein in the molecular weight of Syk (72 kDa). To corroborate the identity of this band, the same membranes were stripped and reprobed with an anti‐Syk polyclonal antibody (Blot anti‐Syk). (B) Averaged data from five independent experiments as that shown in A. Each point represents the mean ± sd of the optical density of the Syk band in the anti‐PY blot.
