Figure 6.
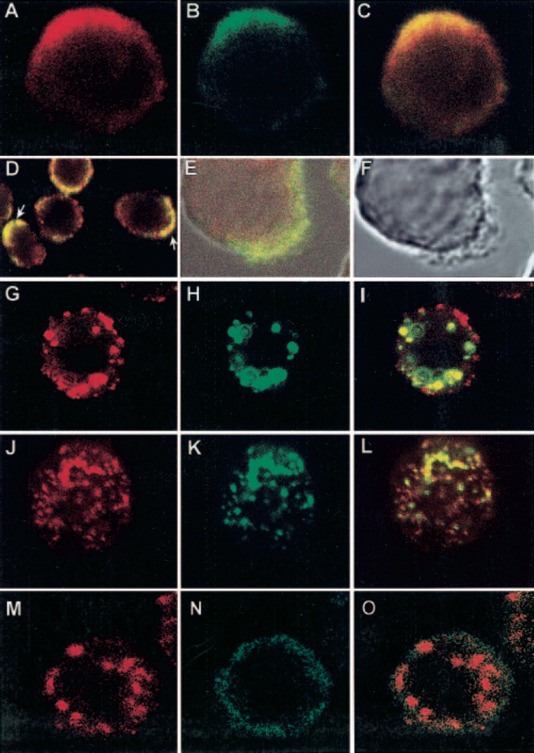
Colocalization and coredistribution of CD13 with FcγRI. (A–D) U‐937 cells were prefixed with PFA. FcγRI was stained first with anti‐FcγRI mAb and FITC‐labeled F(ab)′2 fragments of secondary antibody (B). CD13 was then stained by incubating cells with biotinylated F(ab)′2 fragments of anti‐CD13 mAb and streptavidin‐TR at 4°C (A). (C) Colocalization of FcγRI and CD13 (yellow) in a polarized cell. (D) A field showing CD13 and FcγRI polarized in several cells (arrows). (E, F) Zones of FcγRI and CD13 colocalization correspond to membrane zones showing lamellipodia and fillopodia. (G–I) U‐937 cells were incubated with anti‐FcγRI mAb followed by FITC‐labeled secondary antibody for 20 min at 4°C. Cells were incubated at 37°C for 20 min to allow aggregation, and after fixation, CD13 staining was performed with TR‐labeled F(ab)′2 fragments of anti‐CD13 mAb at 4°C in the presence of sodium azide. (I) Zones of coredistribution of FcγRI and CD13 as yellow aggregates. (J–L) U‐937 cells were incubated with TR‐labeled F(ab)′2 fragments of anti‐CD13 mAb, followed by incubation with a nonlabeled, secondary antibody for 20 min at 4°C and subsequently, at 37°C for 20 min to allow aggregation. After fixation, FcγRI was detected with FITC‐labeled anti‐FcγRI mAb at 4°C in the presence of sodium azide. (L) Coredistribution of FcγRI and CD13. (M–O) U‐937 cells were incubated with anti‐FcγRI mAb followed by phycoerythrin‐labeled secondary antibody for 20 min at 4°C. Cells were incubated at 37°C for 20 min to allow aggregation, and after fixation, CD33 staining was performed with a FITC‐labeled anti‐CD33 mAb at 4°C under the same conditions of CD13 staining in G–I.
