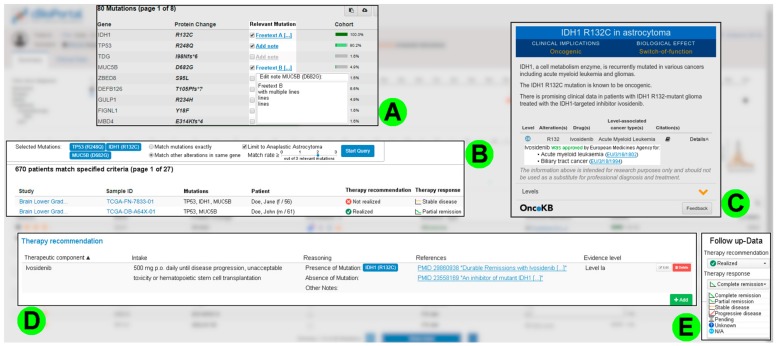
Figure 3.
Collage demonstrating some requirements for the development and recording of a therapy recommendation. This collage depicts image sections from the screenshot mockups we created based on the original interface of cBioPortal: (A) Checkboxes and text fields in the mutation table of the patient view to mark potentially relevant mutations for the therapy recommendation. (B) Search functionality with automatic parameter transfer to find previous patient cases with a mutation pattern similar to that of the current patient. (C) Extension of OncoKB’s information to cover the European Medicines Agency’s (EMA) approval status of a given drug. (D) Summary of already entered components of the therapy recommendation for the current patient. (E) Option to record follow-up data for the current patient. Example data adopted from the public cBioPortal (https://cbioportal.org) and OncoKB [16].