Figure 1.
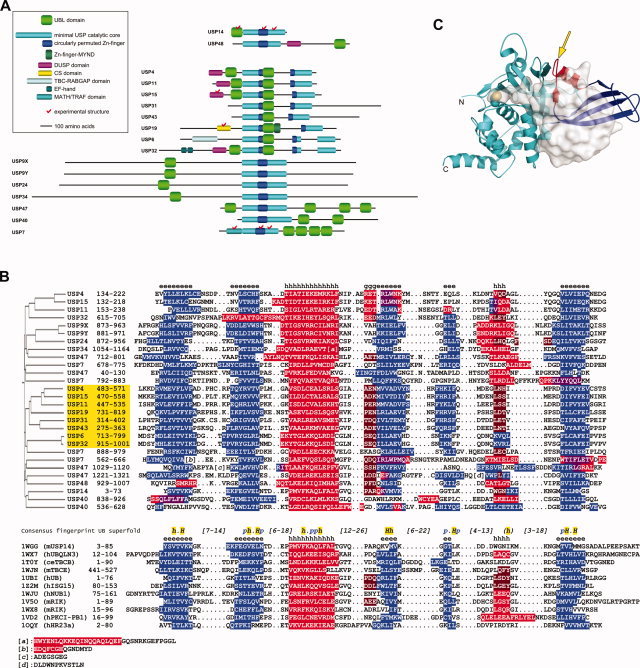
Novel UBL domains of human USPs predicted by structural bioinformatics. (A) Schematic domain organization of human USPs with predicted UBL domains (approximate scale). Note the split in the circularly permuted Zn‐finger‐like domain (blue) and the catalytic core (cyan) due to sizable insertions in some family members. Other domains of these USPs, according to their currently available public annotations, are also shown (see insert). Human USPs 14 and 48, with UBL domains previously annotated based on sequence homology, are shown at the top. (B) Sequence and secondary structure alignment between UBL domains predicted for human USPs (upper part) and selected members from the ubiquitin superfold (lower part, identified primarily by their PDB codes). See Materials and Methods for details, and Supplementary Material for other UBL structures identified by consensus fold recognition as statistically significant templates for the newly detected UBL domains, including the full query‐to‐template assignment. Secondary structure elements, predicted for USP sequences, and observed for the other UBL domains, are highlighted in blue – β‐strand, red – α‐helix, brown – G‐helix, and violet – ambivalent α/β predictions. Consensus secondary structures over the query and template alignments are indicated by e – β‐strand, h – α‐helix, and g – G‐helix. The common fingerprint sequence of the ubiquitin superfold is taken from Ref.29, where conserved hydrophobic residues are denoted by h (90% conserved) and H (100% conserved) and conserved polar residues are denoted by p (90% conserved). A sequence homology‐based clustering of the UBL domains from human USPs is shown on the left. The UBL domains inserted in USP catalytic core domains are highlighted in yellow. (C) Location of UBL domain insertion (arrow) in the minimal USP catalytic core domain, exemplified here from human USP7 (PDB code 1NBF). The papain‐like protease fold is colored in cyan. The nested circularly permuted Zn‐finger‐like domain is in blue, except for its β3‐α1 region (in red) grafted onto the β‐ribbon and attached to the papain‐like domain. The catalytic Cys side chain is rendered as space‐filled model. Bound ubiquitin aldehyde is displayed with translucent molecular surface.
