Figure 3.
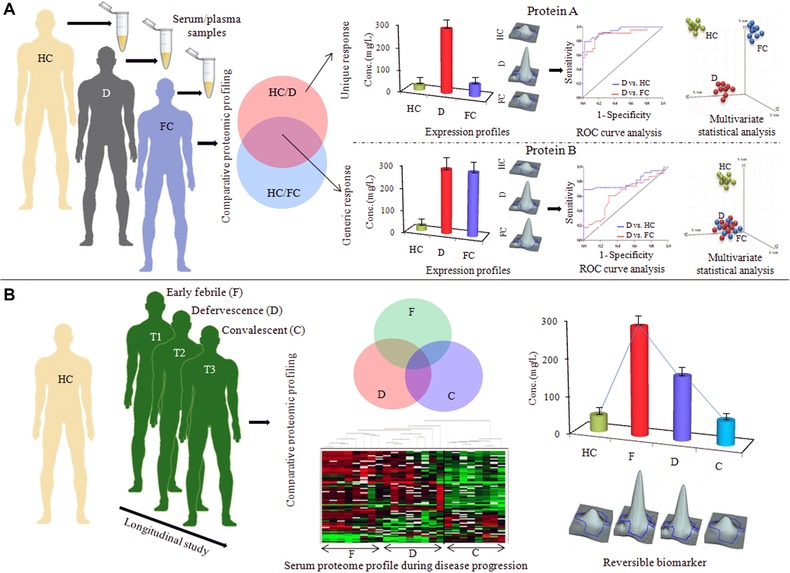
Crucial issues for designing clinical studies for serum/plasma biomarker discovery. (A) Selection of suitable febrile (diseased) controls for evaluating the specificity of the identified markers. Two potential markers (protein A and B) are significantly differentially expressed in an infectious disease population (D) compared to the healthy controls. Between those two candidates, differential expression of protein B is not specific for the disease population (D), it also shows an equal level of altered expression in another closely related infectious disease, which has been used as a febrile control (FC) for the disease D. While the expression level of protein A remained unaltered in the FC population, it showed some extent of specificity toward the disease population D. Downstream analysis of the specificities and sensitivities (ROC curve analysis) and class prediction capabilities of those two potential markers clearly indicates the superiority of the protein A as a potential marker for the disease state D, since it is not only useful in discrimination of disease D from healthy population, but also can successfully differentiate disease D from other closely related clinical manifestations. (B) Analysis of longitudinal cohorts for establishment of prognostic and disease monitoring marker proteins. Information about the reversibility and disease monitoring/prognostic capability of the identified disease surrogates can be obtained from multiple time point analysis (early febrile, defervescence, and convalescent stages of infection).
