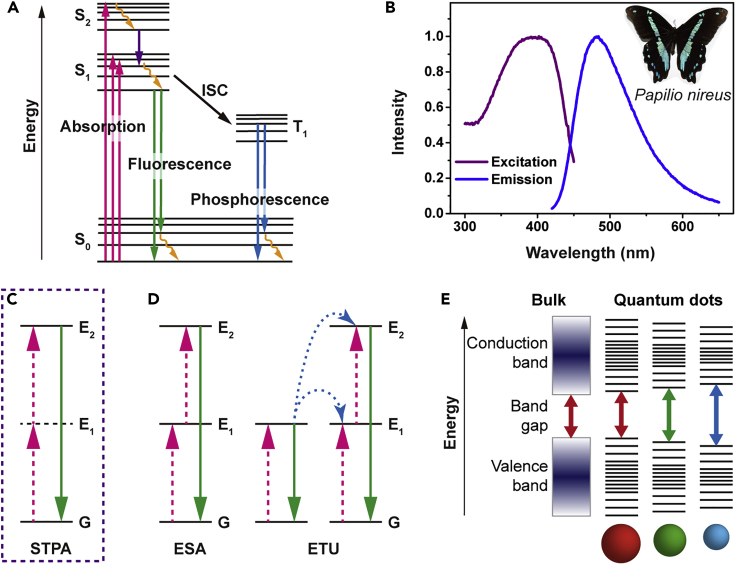
Figure 4.
Fluorescence Excitation and Emission
(A) Jablonski diagram of fluorescence emission. An electron is excited after absorbing a low-energy photon (magenta) and immediately relaxes to the ground state by emitting a low-energy photon (green). It can undergo non-radiative relaxation (yellow). The excited electron can also relax via intersystem crossing (ISC) to a triplet state (T1), which may subsequently relax via phosphorescence (blue) or non-radiative relaxation.
(B) Fluorescence excitation and emission spectra of the blue-green wing scale of the Papilionireus butterfly. Inset: an image of Papilionireus. The wing scales from the colored regions are coated exclusively with fluorescent dyes.
(C) Energy level diagram of the upconversion processes of two-photon dyes: sequential two-photon absorption (STPA). The dashed (magenta) and solid (green) lines represent the photon excitation and emission processes, respectively.
(D) Energy level diagram of the upconversion processes of lanthanide-doped crystals: excited state absorption (ESA) and energy transfer upconversion (ETU). The dashed (magenta), dotted (blue), and solid (green) lines represent the photon excitation, energy conversion, and emission processes, respectively.
(E) Energy level diagram of quantum dots (QDs). The semiconductor band gap is determined by the QD size due to the quantum confinement effect.