Abstract
Objective
The occurrence of non-alcoholic fatty liver disease (NAFLD) is globally increasing. To challenge the current incidence of NAFLD, non-invasive markers that could identify patients at risk or monitor disease progression are an important need. Copper intake and organ copper concentrations have earlier been linked to NAFLD progression, but serum copper does not adequately represent the disease state. Cu atoms occur under the form of two stable isotopes, 63Cu and 65Cu, and the ratio of both (expressed as δ65Cu, in ‰) in blood serum has been shown to be altered in chronic liver disease. To assess whether the Cu isotope ratio might predict disease occurrence and progression of NAFLD, the serum Cu isotopic composition of patients with different stages of NAFLD was determined.
Results
Our results showed that serum δ65Cu values were lower in NAFLD patients, already at the level of simple steatosis, and remained stable during further disease progression. ROC analysis shows an almost perfect diagnostic ability of serum δ65Cu values for NAFLD, but no discrimination between different severity degrees could be made. Therefore, the serum Cu isotopic composition might show potential for early diagnosis of NAFLD patients.
Keywords: Liver steatosis, Non-alcoholic steatohepatitis, Cu isotopes, Multi collector inductively coupled plasma mass spectrometry
Introduction
Non-alcoholic fatty liver disease (NAFLD) is the most common liver disease with a global current prevalence of 24% [1]. In the past two decades, its incidence has increased about fivefold, especially in young adults [2]. NAFLD can range from simple hepatic steatosis (fat accumulation, non-alcoholic fatty liver, NAFL) to liver inflammation (non-alcoholic steatohepatitis, NASH), which can evolve to liver fibrosis with varying degree of liver dysfunction [3]. Since patients with steatohepatitis are at increased risk of fibrosis development and might even develop hepatocellular carcinoma (HCC), and no pharmacological option for any stage of NAFLD is available so far, it is of interest to detect patients early in disease progression.
Recently, there has been a growing interest in high-precision Cu isotopic analysis, i.e. evaluating the 65Cu/63Cu ratio (expressed as δ65Cu, in ‰), as a diagnostic and prognostic tool for liver disease [4–7]. 63Cu and 65Cu are the two stable Cu isotopes that occur naturally, showing small, but systematic variations in their abundance ratio under certain disease conditions that can be reliably quantified [8–10]. It has been published recently that patients destined for bariatric surgery may show lower serum δ65Cu values [11]. Bariatric surgery is performed on morbidly obese patients to reduce food intake and obtain weight loss, and NAFLD is a prominent health risk in these patients. In the latter study, no liver biopsies were taken and no fibroscan was used to assess NAFLD in this patient cohort, and thus the initial status of liver disease was not known.
Therefore, we have investigated the diagnostic potential of serum δ65Cu values for different stages of NAFLD. We have collected serum from patients with biopsy-proven NAFLD with varying severity, and subjected these samples to high-precision Cu isotopic analysis via multi-collector inductively coupled plasma-mass spectrometry (MC-ICP-MS).
Main text
Materials and methods
Patient cohort
A patient cohort of 10 NAFL and 14 NASH patients was recruited at the Ghent University Hospital within the context of a previous study (Table 1 for patient characteristics). Non-cirrhotic patients were scheduled for bariatric surgery. Specific clinical, biochemical, histological and/or radiographic criteria were used to exclude liver disease of other aetiologies, including cholestatic, alcohol-induced or drug-induced liver disease and viral or auto-immune hepatitis. All patients had biopsy-confirmed NAFLD and a negative history of alcohol abuse (average daily consumption of less than 20 g EtOH). Healthy controls from previous studies were used for comparison (Table 1) [11–13].
Table 1.
Clinical characteristics of the patient cohort. Results are expressed as mean ± 95% CI
| Characteristic | NAFL (n = 10) | NASH F0-F2 (n = 10) | NASH F4 (n = 4) |
|---|---|---|---|
| Age (years) | 39.8 ± 7.8 | 47.5 ± 8.9 | 66.8 ± 17.1 |
| Gender (M %/F %) | 10/90 | 0/100 | 100/0 |
| BMI (kg m−2) | 41.6 ± 6.5 | 42.7 ± 7.2 | 29.0 ± 11.2 |
| Type 2 diabetes (%) | 10 | 30 | 100 |
| AST (U l−1) | 23.3 ± 4.2 | 32.6 ± 17.0 | 31.8 ± 21.5 |
| ALT (U l−1) | 24.2 ± 5.4 | 39.4 ± 24.3 | 33.3 ± 11.7 |
| GGT (U l−1) | 16.0 ± 4.2 | 51.5 ± 27.8 | 139.5 ± 142.6 |
| Platelets (103 µl−1) | 274.5 ± 29.9 | 297.3 ± 64.1 | 133.8 ± 87.0 |
| Hb (g dl−1) | 13.2 ± 0.6 | 13.5 ± 1.3 | 12.4 ± 3.4 |
| HbA1c (%) | 5.6 ± 0.3 | 6.1 ± 1.2 | 6.2 ± 1.8 |
| Total cholesterol (mg dl−1) | 186.8 ± 21.1 | 195.4 ± 40.0 | |
| LDL (mg dl−1) | 94.2 ± 16.3 | 103.2 ± 21.0 | |
| HDL (mg dl−1) | 66.3 ± 11.8 | 44.4 ± 13.0 | |
| Triglycerides (mg dl−1) | 121.7 ± 26.7 | 219.9 ± 76.5 | |
| Bilirubin (mg dl−1) | 0.4 ± 0.1 | 0.4 ± 0.1 | |
| Fasting glucose (mg dl−1) | 98.2 ± 18.5 | 97.5 ± 9.6 | |
| HOMA-IR | 4.4 ± 2.4 | 6.9 ± 2.9 | |
| Iron (µg dl−1) | 76.6 ± 26.4 | 66.3 ± 19.0 | |
| Transferrin (mg dl−1) | 2.9 ± 0.3 | 2.6 ± 0.7 | |
| Ferritin (µg l−1) | 60.0 ± 38.6 | 155.1 ± 122.3 | |
| Copper (µg dl−1) | 137.4 ± 38.4 | 117.3 ± 39.6 |
| Control population | |||
|---|---|---|---|
| REF | Age, years | Gender (M%/F%) | n |
| [11] | 43.8 ± 8.5 | 0/100 | 10 |
| [12] | 23–48 | 100/0 | 5 |
| [13] | 51.57 ± 6.9 | 100/0 | 7 |
NAFL non-alcoholic fatty liver, NASH non-alcoholic steatohepatitis, BMI body mass index, AST aspartate aminotransferase, ALT alanine aminotransferase, GGT γ-glutamyltransferase, Hb Haemoglobin, HbA1c haemoglobin A1c, LDL low-density lipoprotein, HDL high-density lipoprotein, HOMA-IR Homeostasis Model Assessment Insulin Resistance
Clinical evaluation
Anthropometric measurements of all patients were taken and the body mass index (BMI) was calculated as body weight/height2 (kg m−2). Blood samples from patients were collected, centrifuged (10 min, 4 °C, 350 g), and serum was stored at − 80 °C until analysis. Thawed serum was analysed for the levels of aspartate aminotransferase (AST), alanine aminotransferase (ALT), γ-glutamyl transpeptidase (GGT), bilirubin, haemoglobin, triglycerides, high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol, total serum cholesterol, fasting glucose, haemoglobin A1c (HbA1c) and insulin and complete blood count. Insulin resistance was estimated by the homeostasis model assessment-insulin resistance (HOMA-IR) [14], and the presence of diabetes mellitus (according to the American Diabetes Association criteria [15]) was evaluated. In addition, iron, transferrin, ferritin and copper serum levels were determined.
Formalin-fixed liver biopsies were routinely processed and stained with haematoxylin and eosin, and Masson’s trichrome. All biopsies were blinded and evaluated by an experienced pathologist according to the NASH clinical research network scoring system [16].
Serum isotopic analysis
Serum samples (400 µL), were thawed and digested in perfluoroalkoxy (PFA) digestion vessels (Savillex, USA) with a 2:1 v/v mixture of 14 M pro analysis HNO3 (Chem-lab, Belgium and additionally purified by means of sub-boiling distillation) and 9.8 M H2O2 (Sigma-Aldrich, Belgium). The digestion was performed at 110 °C for 18 h. The digest was then dried at 90 °C and the solid residue was re-dissolved in 5 mL of 8 M Optima™ grade HCl (Fisher Chemical, UK) and 0.001% H2O2 to ensure that Cu is present in its Cu(II) oxidation state.
Cu was isolated by anion exchange chromatography using 1 mL of AG-MP-1 resin (Bio-Rad Laboratories, CA, USA) [12]. Two column passes ensure sufficient purity, while a quantitative recovery avoids potential on-column isotope fractionation effects from affecting the final Cu isotope ratio results. The purified Cu fractions thus obtained were dried at 90 °C and the residue was re-dissolved in 1 mL of 14 M HNO3. This was done twice to fully remove remaining chlorine. Finally, the Cu fractions were re-dissolved in 0.42 M HNO3 for further elemental and isotopic analysis. Ultra-pure water (resistivity > 18.2 MΩ cm) was used throughout this study (Milli-Q Element water purification system, Millipore, France). All sample manipulations were performed in a class-10 clean room.
Cu isotope ratio measurements were performed using a Neptune MC-ICP-MS instrument (Thermo Scientific, Germany), as described elsewhere [5]. Samples were measured in sample-standard bracketing (SSB) sequence using a solution of NIST SRM 976 Cu isotopic reference material (NIST, USA) as external standard. Cu concentrations in samples and standard were matched within ± 10%. The mass bias was corrected for using a combination of internal standardisation using Ga as internal standard, relying on the revised Russell’s law as described by Baxter et al. [17], and external correction in an SSB approach. The Cu isotope ratio is expressed in delta notation as δ65Cu (‰), calculated as indicated in Eq. 1:
| 1 |
A Cu in-house standard (Inorganic Ventures, the Netherlands) was measured every five samples to monitor the quality of the isotope ratio data. The Cu isotope ratio obtained for the in-house QC sample in this work was δ65Cu = 0.223 ± 0.009 (sd, n = 9), which corresponds well with the value obtained in our previous study [12].
Statistics
Statistical analysis was performed using Graphpad Prism (Graphpad Software Inc., San Diego, California) and SPSS Statistics 25 (IBM Corp., USA). Kruskall-Wallis and Dunn’s multiple comparisons test were used to compare groups and Receiver Operating Characteristic (ROC) analysis was performed. Spearman’s rank correlation coefficient was used to evaluate potential relationships between biochemical parameters and the Cu isotopic composition within the NAFLD population. P-values were two-sided and considered significant when lower than 0.05.
Results
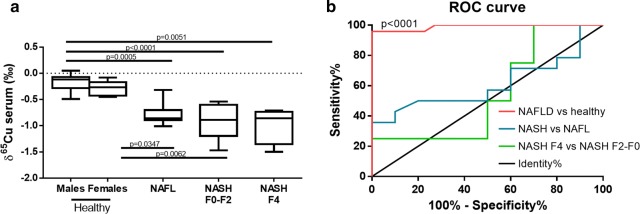
In NAFLD patients, serum δ65Cu values were lower than in healthy controls (Fig. 1a), even at the level of simple steatosis (NAFL) (p ≤ 0.04), and remained stable during further disease progression (Fig. 1a). More specifically, average serum δ65Cu values were lower by 0.57‰ ± 0.35‰ (sd), 0.71 ± 0.47‰ (sd), and 0.76 ± 0.51‰ (sd) in NAFL patients, NASH patients with F0–F2 fibrosis, and NASH patients with cirrhosis (F4), respectively. This suggests that the lower serum δ65Cu value is an early event in NAFLD and is thus already present before the onset of liver inflammation.
Fig. 1.
Serum δ65Cu changes in non-alcoholic fatty liver disease (NAFLD). a Serum δ65Cu values for different stages of NAFLD. Statistical analysis was performed by Kruskal–Wallis and Dunn’s multiple comparisons test. b Receiver Operating Characteristic (ROC) analysis of serum δ65Cu values. P-values were two-sided and considered significant when lower than 0.05. F0–F4 refers to the corresponding fibrosis stage. Healthy males: n = 12, healthy females: n = 10. Non-alcoholic fatty liver (NAFL): n = 10. Non-alcoholic steatohepatitis (NASH) F0–F2: n = 10. NASH F4: n = 4
Importantly, ROC analysis shows an almost complete (AUC = 0.9896, p < 0.0001) diagnostic ability of serum δ65Cu values for NAFLD patients (Fig. 1b). In contrast, no further discrimination can be made between NAFL and NASH patients (AUC = 0.6107, p = 0.36) or between NASH patients with F0–F2 fibrosis and with cirrhosis (F4) (AUC = 0.5500, p = 0.78). In addition, no relevant or significant correlations between clinical parameters and serum δ65Cu values within the NAFLD patient cohort were observed (max |ρ| = 0.22, Table 2).
Table 2.
Spearman’s correlation analysis between serum δ65Cu levels and clinical parameters within the NAFLD patient cohort
| Spearman’s ρ | p | n | |
|---|---|---|---|
| BMI (kg m−2) | 0.03 | 0.89 | 24 |
| Steatosis on liver biopsy | − 0.22 | 0.34 | 21 |
| Ballooning on liver biopsy | − 0.20 | 0.37 | 21 |
| Inflammation on liver biopsy | − 0.09 | 0.71 | 21 |
| NAS on liver biopsy | − 0.19 | 0.42 | 21 |
| Fibrosis on liver biopsy | − 0.13 | 0.54 | 24 |
| Presence of NASH (yes/no) | − 0.19 | 0.38 | 24 |
| AST (U l−1) | 0.25 | 0.23 | 24 |
| ALT (U l−1) | 0.03 | 0.89 | 24 |
| GGT (U l−1) | − 0.05 | 0.81 | 24 |
| Bilirubin (mg dl−1) | − 0.07 | 0.77 | 20 |
| Glucose (mg dl−1) | 0.24 | 0.30 | 20 |
| HOMA-IR | 0.26 | 0.27 | 20 |
| HbA1c (%) | 0.09 | 0.70 | 23 |
| Diabetes (yes/no) | 0.19 | 0.39 | 24 |
| Cholesterol (mg dl−1) | 0.22 | 0.39 | 17 |
| HDL (mg dl−1) | − 0.10 | 0.71 | 17 |
| LDL (mg dl−1) | − 0.06 | 0.80 | 19 |
| Triglycerides (mg dl−1) | − 0.16 | 0.50 | 20 |
| Ferritin (µg l−1) | 0.24 | 0.33 | 19 |
| Copper (µg dl−1) | 0.10 | 0.69 | 18 |
| Transferrin (mg dl−1) | − 0.03 | 0.91 | 19 |
| Iron (µg dl−1) | − 0.03 | 0.92 | 19 |
NAFL non-alcoholic fatty liver, NASH: non-alcoholic steatohepatitis, BMI: body mass index, AST aspartate aminotransferase, ALT alanine aminotransferase, GGT γ-glutamyltransferase, Hb Haemoglobin, HbA1c haemoglobin A1c, LDL low-density lipoprotein, HDL high-density lipoprotein, HOMA-IR Homeostasis Model Assessment Insulin Resistance, NAS NAFLD activity score
Discussion
Lower serum δ65Cu values have been described in patients with end stage liver disease (cirrhosis), arising from several aetiologies, in cancer patients, and in patients with Wilson’s disease [4–8]. In addition, we have shown a whole-body enrichment of the light 63Cu isotope in an experimental model for secondary biliary fibrosis [18]. Our data align those previous results and further elaborate on serum δ65Cu values within different NAFLD stages. In agreement with previously published data of a bariatric cohort [11], serum δ65Cu values were lower in NAFLD patients. The variation of serum δ65Cu values in different stages of NAFLD had not been investigated thus far, and we show that, already at the level of simple steatosis, serum δ65Cu values are lower, and remain stable during further disease progression. We highlight lower serum δ65Cu values as diagnostic tool for NAFLD, irrespective of disease progression. Currently, patients are screened for steatosis by non-invasive imaging techniques (such as ultrasound or the controlled attenuation parameter), which require patients to go to a hospital or center where this equipment is available [19]. In addition, the general practitioner can take blood samples for serum biomarker analysis (such as the fatty liver index or the SteatoTest). However, the diagnostic ability of these biomarkers for steatosis (AUROC ranging from 0.73–0.86) can still be improved [19]. Our results show potential for serum δ65Cu to exceed the diagnostic ability of current serum biomarkers, and to diagnose patients with early NAFLD, a population that would benefit from early notification to prevent progression to NASH, for which no medical therapy is available so far.
Limitations
Although our patient population covers different stages of NAFLD, no obese patients without NAFLD were included. These patients could have revealed additional information about the initial stage and cause of the lower serum δ65Cu values. In addition, both genders were not equally represented in the different NAFLD stages. However, when we compare male and female healthy controls, the difference between average serum δ65Cu values was much smaller (0.12‰) than the observed disease effects (0.57–0.76‰) and not significant (Fig. 1a). A larger patient cohort could have also allowed to evaluate assay reproducibility and sensitivity. Nevertheless, serum δ65Cu values have previously been shown to be reproducible in healthy controls [11–13], so that these results seem highly reliable.
Acknowledgements
Not applicable.
Abbreviations
- ALT
Alanine aminotransferase
- AST
Aspartate aminotransferase
- BMI
Body mass index
- GGT
γ-Glutamyltransferase
- Hb
Haemoglobin
- HbA1c
Haemoglobin A1c
- HCC
Hepatocellular carcinoma
- HDL
High-density lipoprotein
- HOMA-IR
Homeostasis Model Assessment Insulin Resistance
- LDL
Low-density lipoprotein
- NASH
Non-alcoholic steatohepatitis
- NAFL
Non-alcoholic fatty liver
- NAFLD
Non-alcoholic fatty liver disease
Authors’ contributions
SVC, AAMBH, LD, HVV, FV and MCR conceptualized and designed the experiment and methodology. SVC and SL performed sample collection, data acquisition and analysis under the supervision of LD and HVV. AH performed the MC-ICP-MS Cu isotopic analysis under supervision of MCR and FV. SVC, AAMBH and LD wrote the manuscript. All authors contributed to data interpretation and to the final manuscript.
Funding
The Special Research Fund of Ghent University BOF-UGent is acknowledged for financial support under the form of an interdisciplinary research project (IOP). MCR thanks FWO-Vlaanderen for her post-doctoral grant. HVV is an FWO senior researcher.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding authors on reasonable request.
Ethics approval and consent to participate
The study was approved by the Ghent University Hospital Ethical Committee and all participants gave their written informed consent at Ghent University Hospital.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Sanne Van Campenhout and Agustina A. M. B. Hastuti contributed equally to this work
Marta Costas-Rodríguez and Lindsey Devisscher contributed equally to this work
Contributor Information
Sanne Van Campenhout, Email: Sanne.VanCampenhout@UGent.be.
Agustina A. M. B. Hastuti, Email: Agustina.Hastuti@UGent.be
Sander Lefere, Email: Sander.Lefere@UGent.be.
Hans Van Vlierberghe, Email: Hans.VanVlierberghe@UGent.be.
Frank Vanhaecke, Email: Frank.Vanhaecke@UGent.be.
Marta Costas-Rodríguez, Email: marta.costasrodriguez@UGent.be.
Lindsey Devisscher, Email: lindsey.devisscher@UGent.be.
References
- 1.Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15:11–20. doi: 10.1038/nrgastro.2017.109. [DOI] [PubMed] [Google Scholar]
- 2.Allen AM, Therneau TM, Larson JJ, Coward A, Somers VK, Kamath PS. Nonalcoholic fatty liver disease incidence and impact on metabolic burden and death: a 20 year-community study. Hepatology. 2018;67:1726–1736. doi: 10.1002/hep.29546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calzadilla Bertot L, Adams LA. The natural course of non-alcoholic fatty liver disease. Int J Mol Sci. 2016 doi: 10.3390/ijms17050774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Costas-Rodríguez M, Anoshkina Y, Lauwens S, Van Vlierberghe H, Delanghe J, Vanhaecke F. Isotopic analysis of Cu in blood serum by multi-collector ICP-mass spectrometry: a new approach for the diagnosis and prognosis of liver cirrhosis? Metallomics. 2015;7:491–498. doi: 10.1039/C4MT00319E. [DOI] [PubMed] [Google Scholar]
- 5.Lauwens S, Costas-Rodríguez M, Van Vlierberghe H, Vanhaecke F. Cu isotopic signature in blood serum of liver transplant patients: a follow-up study. Sci Rep. 2016;6:30683. doi: 10.1038/srep30683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Balter V, Nogueira da Costa A, Bondanese VP, Jaouen K, Lamboux A, Sangrajrang S, et al. Natural variations of copper and sulfur stable isotopes in blood of hepatocellular carcinoma patients. Proc Natl Acad Sci. 2015;112:982–985. doi: 10.1073/pnas.1415151112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aramendía M, Rello L, Resano M, Vanhaecke F. Isotopic analysis of Cu in serum samples for diagnosis of Wilson’s disease: a pilot study. J Anal At Spectrom. 2013;28:675. doi: 10.1039/c3ja30349g. [DOI] [Google Scholar]
- 8.Télouk P, Puisieux A, Fujii T, Balter V, Bondanese VP, Morel A-P, et al. Copper isotope effect in serum of cancer patients. A pilot study. Metallomics. 2015;7:299–308. doi: 10.1039/C4MT00269E. [DOI] [PubMed] [Google Scholar]
- 9.Costas-Rodríguez M, Delanghe J, Vanhaecke F. High-precision isotopic analysis of essential mineral elements in biomedicine: natural isotope ratio variations as potential diagnostic and/or prognostic markers. TrAC Trends Anal Chem. 2016;76:182–193. doi: 10.1016/j.trac.2015.10.008. [DOI] [Google Scholar]
- 10.Albarede F, Télouk P, Balter V, Bondanese VP, Albalat E, Oger P, et al. Medical applications of Cu, Zn, and S isotope effects. Metallomics. 2016;8:1056–1070. doi: 10.1039/C5MT00316D. [DOI] [PubMed] [Google Scholar]
- 11.Hastuti AAMB, Costas-Rodríguez M, Anoshkina Y, Parnall T, Madura JA, Vanhaecke F. High-precision isotopic analysis of serum and whole blood Cu, Fe and Zn to assess possible homeostasis alterations due to bariatric surgery. Anal Bioanal Chem. 2020;412:727–738. doi: 10.1007/s00216-019-02291-2. [DOI] [PubMed] [Google Scholar]
- 12.Lauwens S, Costas-Rodríguez M, Van Vlierberghe H, Vanhaecke F. High-precision isotopic analysis of Cu in blood serum via multi-collector ICP-mass spectrometry for clinical investigation: steps towards improved robustness and higher sample throughput. J Anal At Spectrom. 2017;32:597–608. doi: 10.1039/C6JA00433D. [DOI] [Google Scholar]
- 13.Lauwens S, Costas-Rodríguez M, Delanghe J, Van Vlierberghe H, Vanhaecke F. Quantification and isotopic analysis of bulk and of exchangeable and ultrafiltrable serum copper in healthy and alcoholic cirrhosis subjects. Talanta. 2018;189:332–338. doi: 10.1016/j.talanta.2018.07.011. [DOI] [PubMed] [Google Scholar]
- 14.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and?-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 15.Model CC Standards of medical care in diabetes—2015 abridged for primary care providers. Clin Diabetes. 2015;33:97–111. doi: 10.2337/diaclin.33.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 17.Baxter DC, Rodushkin I, Engström E, Malinovsky D. Revised exponential model for mass bias correction using an internal standard for isotope abundance ratio measurements by multi-collector inductively coupled plasma mass spectrometry. J Anal At Spectrom. 2006;21:427. doi: 10.1039/b517457k. [DOI] [Google Scholar]
- 18.Costas-Rodríguez M, Van Campenhout S, Hastuti AAMB, Devisscher L, Van Vlierberghe H, Vanhaecke F. Body distribution of stable copper isotopes during the progression of cholestatic liver disease induced by common bile duct ligation in mice. Metallomics. 2019 doi: 10.1039/C8MT00362A. [DOI] [PubMed] [Google Scholar]
- 19.European Association for the Study of the Liver (EASL) EASL–EASD–EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. Diabetologia. 2016;59:1121–1140. doi: 10.1007/s00125-016-3902-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding authors on reasonable request.