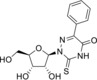
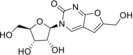
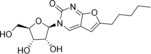
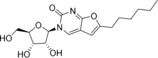
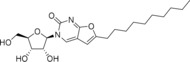
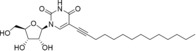
Table 1.
Structures, toxicity, and antiviral activity of nucleoside analogues.
|
Structure |
PEK CC50 [μM][a] |
RD CC50 [μM][b] |
TBEV EC50 [μM] |
EV EC50 [μM][c] |
|---|---|---|---|---|
|
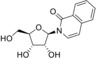
1‐(β‐d‐ribofuranosyl)isocarbostyryl (1) |
>50 (24 h); >50 (7 d) |
>125 (24 h); 73 (7 d) |
> 50 |
> 125 (EVA71, CVB1, PV1) |
|
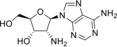
2’‐Amino‐2’‐deoxyadenosine (2) |
>50 (24 h); 26 (7 d) |
73 (24 h); 20 (7 d) |
> 50 |
> 125 (EVA71, CVB1, PV1) |
|
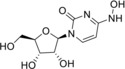
N 4‐Hydroxycytidine (3) |
>50 (24 h); >50 (7 d) |
73 (24 h); 73 (7 d) |
> 50 |
28±13 (EVA71); 5.41 (CVA16); 18.41 (CVA9); 7.74 (CVB1); 18.41 (ECHO30); 73.66 (ECHO6); 36.83 (PV1) |
|
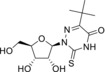
2‐Thio‐5‐(tert‐butyl)‐6‐azauridine (4) |
NDa |
104 (24 h); 104 (7 d) |
> 50 |
> 125 (EVA71, CVB1, PV1) |
|
2‐Thio‐5‐phenyl‐6‐azauridine (5) |
ND |
>125 (24 h); >125 (7 d) |
> 50 |
> 125 (EVA71, CVB1, PV1) |
|
3‐(β‐d‐Ribofuranosyl)‐6‐hydroxymethyl‐2,3‐dihydrofurano[2, 3‐d]pyrimidin‐2‐one (6) |
ND |
>125 (24 h); >125 (7 d) |
> 50 |
> 125 (EVA71, CVB1, PV1) |
|
3‐(β‐d‐Ribofuranosyl)‐6‐pentyl‐2,3‐dihydrofurano[2, 3‐d]pyrimidin‐2‐one (7) |
ND |
73 (24 h); 73 (7 d) |
> 50 |
18±12 (EVA71); 4.6 (CVA16); > 125 (CVA9, CVB1, ECHO30, ECHO6, PV1) |
|
3‐(β‐d‐Ribofuranosyl)‐6‐hexyl‐2,3‐dihydrofurano[2, 3‐d]pyrimidin‐2‐one (8) |
ND |
73 (24 h); 36 (7 d) |
> 50 |
16±9 (EVA71); 3.26 (CVA16); > 125 (CVA9, CVB1, ECHO30, ECHO6, PV1) |
|
3‐(β‐d‐Ribofuranosyl)‐6‐decyl‐2,3‐dihydrofurano[2, 3‐d]pyrimidin‐2‐one (9) |
>50 (24 h); >50 (7 d) |
>125 (24 h); >125 (7 d) |
> 50 |
> 125 (EVA71, CVB1, PV1) |
|
5‐(Tetradec‐1‐yn‐1‐yl)‐uridine (10) |
>50 (24 h); >50 (7 d) |
73 (24 h); 20 (7 d) |
9.4±0.4 |
> 73 (EVA71, CVB1, PV1) |
|
N 6‐Benzyladenosine (12 a) |
ND |
9.21 (24 h); 9.21 (7 d) |
> 50 |
2.5±0.2 (EVA71); 0.92±0.24 (CVA16); 7.75±3.15 (CVB1); 11.1±1.9 (ECHO30); 10.15±0.95 (PV1) |
|
dUY11 (11 a) |
>50 (24 h); >50 (7 d)[e] |
ND |
0.024±0.013[e] |
ND |
[a] PEK, porcine embryo kidney cells. [b] RD, rhabdomyosarcoma cells. [c] EV, enteroviruses. [d] ND, not determined. [e] Data from ref. 12.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.