Abstract
Objective
The Middle East respiratory syndrome coronavirus (MERS‐CoV) emerged in 2012 on the Arabian Peninsula and has caused severe respiratory disease with more than 800 laboratory‐confirmed cases. The return of infected pilgrims to their home countries with a putative spread of MERS‐CoV necessitates further surveillance.
Methods
A cross sectional study of 839 adult African Hajj pilgrims returning to Accra in Ghana, West Africa, was conducted in 2013 to assess the prevalence of respiratory symptoms as well as of MERS‐CoV, human rhinovirus (HRV), respiratory syncytial virus (RSV) and influenza A virus (FLU A) infection.
Results
Six hundred and fifty‐one (77.6%) pilgrims had respiratory symptoms. Tests were positive for at least one of the viruses other than MERS‐CoV in 179 (21.3%) of all pilgrims, with 22.4% detection in symptomatic vs. 17.6% detection in asymptomatic pilgrims. No MERS‐CoV was detected, although common respiratory viruses were prevalent, with positive findings for HRV in 141 individuals (16.8%), RSV in 43 individuals (5.1%) and FLU A in 11 individuals (1.3%). Results were positive for more than one virus in 16 (1.9%) individuals, including 14 (1.7%) RSV/HRV co‐infections and 2 (0.2%) FLU A/HRV co‐infections. A total 146 (22.4%) of the symptomatic returnees tested positive for at least one respiratory virus compared with 33 (17.6%) of the asymptomatic pilgrims who had at least one detectable virus in their sample.
Conclusions
The prevalence of viral respiratory infections among Hajj pilgrims in both symptomatic and asymptomatic subjects was high. Although it is reassuring that MERS‐CoV was not detected in the tested population, there is a need for active surveillance of Hajj pilgrims.
Keywords: MERS‐coronavirus, respiratory illness, Hajj pilgrimage, Human rhinovirus, Respiratory syncytial virus, Influenza A virus
Abstract
Objectif
Le syndrome respiratoire à coronavirus du Moyen‐Orient (MERS‐CoV) est apparu en 2012 sur la péninsule arabique et a causé une maladie respiratoire sévère avec plus de 800 cas confirmés de laboratoire. Le retour des pèlerins infectés dans leurs pays d'origine avec la possibilité de propagation du MERS‐CoV nécessite une surveillance accrue.
Méthodes
Une étude de surveillance de 839 pèlerins du Hajj, adultes africains, de retour à Accra au Ghana, en Afrique de l'Ouest, a été menée en 2013 pour évaluer la prévalence des symptômes respiratoires ainsi que des infections à MERS‐CoV, au rhinovirus humain (RVH), au virus respiratoire syncytial (VRS) et au virus de l'influenza A (FLU A).
Résultats
651 (77,6%) pèlerins avaient des symptômes respiratoires. Les tests étaient positifs pour au moins un des virus autres que MERS‐CoV chez 179 (21,3%) de tous les pèlerins, avec une détection de 22,4% chez les pèlerins symptomatiques versus 17,6% chez les asymptomatiques. Aucun cas MERS‐CoV n'a été détecté, bien que les virus respiratoires communs fussent répandus, avec des résultats positifs pour le RVH chez 141 personnes (16,8%), le VRS chez 43 individus (5,1%) et le FLU A chez 11 sujets (1,3%). Les résultats ont été positifs pour plus d'un virus chez 16 (1,9%) personnes, dont 14 (1,7%) coinfections VRS/ RVH et 2 (0,2%) coinfections FLU A/RVH. Au total, 146 (22,4%) pèlerins symptomatiques ont testé positif pour au moins un virus respiratoire comparé à 33 (17,6%) pèlerins asymptomatiques qui avaient au moins un virus détectable dans leur échantillon.
Conclusions
La prévalence des infections respiratoires virales chez les pèlerins du Hadj symptomatiques et asymptomatiques est élevée. Même s'il est rassurant de constater que MERS‐CoV n'a pas été détecté dans la cohorte testée, une surveillance active des pèlerins du Hadj est nécessaire.
Keywords: MERS‐Coronavirus, maladie respiratoire, pèlerinage du Hajj, rhinovirus humain, virus respiratoire syncytial, virus de l'influenza A
Abstract
Objetivo
El coronavirus del Síndrome Respiratorio de Oriente Medio (MERS‐CoV) surgió en el 2012 en la Península Arábiga y ha causado una enfermedad respiratoria severa con más de 800 casos confirmados en laboratorio. El regreso de peregrinos infectados a sus países de origen con una supuesta propagación del MERS‐CoV requiere de mayor vigilancia.
Métodos
En el 2013 se realizó un estudio de vigilancia de 839 adultos Africanos, peregrinos Hajj que regresaban a Accra en Ghana, África Occidental, con el fin de evaluar la prevalencia de síntomas respiratorios al igual que la presencia de MERS‐CoV, Rinovirus humano (RVH), Virus Sincicial Respiratorio (VSR), e infección por el virus Influenza A (FLU A).
Resultados
651 (77.6%) peregrinos presentaban síntomas respiratorios. Las pruebas eran positivas para al menos uno de los virus, diferente del MERS‐CoV, en 179 (21.3%) de los peregrinos, con una detección del 22.4% en peregrinos sintomáticos vs. 17.6% en peregrinos asintomáticos. No se detectó MERS‐CoV, siendo más prevalentes los virus respiratorios comunes, con hallazgos positivos para RVH en 141 individuos (16.8%), VSR en 43 individuos (5.1%), y FLU A en 11 individuos (1.3%). Los resultados eran positivos para más de un virus en 16 (1.9%) individuos, incluyendo 14 (1.7%) coinfecciones VSR /RVH y 2 (0.2%) coinfecciones FLU A/RVH. Un total de 146 (22.4%) de los viajeros sintomáticos dieron positivo para al menos un virus respiratorio, comparados con 33 (17.6%) de los peregrinos asintomáticos que tenían al menos un virus detectable en su muestra.
Conclusiones
La prevalencia de las infecciones respiratorias virales es alta en peregrinos Hajj sintomáticos y asintomáticos. Aunque es alentador que no se haya detectado MERS‐CoV en la cohorte evaluada, es necesario mantener una vigilancia activa para peregrinos Hajj.
Introduction
Coronaviruses (CoV) in the genera Alphacoronavirus and Betacoronavirus [order Nidovirales, family Coronaviridae, subfamily Coronavirinae] infect a broad range of mammalian species including humans 1. While the human CoVs (HCoV)‐HKU1, HCoV‐229E, HCoV‐NL63 and HCoV‐OC43 cause mild to moderate respiratory tract infection, an epidemic of severe lower respiratory tract infections with a case fatality rate of approximately 10% was caused by Severe Acute Respiratory Syndrome‐CoV (SARS‐CoV) in 2002–2004 2. In 2012, a novel coronavirus termed Middle East respiratory syndrome‐CoV (MERS‐CoV) emerged on the Arabian Peninsula. MERS‐CoV was found in more than 800 cases, with a steep increase case numbers in early 2014 3, 4, 5. Clinically, MERS‐CoV presents mainly as a respiratory disease, with symptoms ranging from asymptomatic or mild upper respiratory tract disease to severe viral pneumonia and multi‐organ failure. While most confirmed MERS‐CoV cases originate from the Arabian Peninsula, several MERS‐CoV infections have been imported to Europe (United Kingdom, Germany, Italy, France, Greece, Netherlands), Asia (Malaysia, Philippines) and the United States of America 6, 7, 8, 9, 10, 11, 12, 13.
All cases detected outside the Arabian Peninsula were linked to a Middle Eastern country either as a direct result of recent travel or through person‐to‐person transmission via a contact case. These imported cases highlight the fact that MERS‐CoV, like other airborne viruses such as influenza, has the capability for worldwide spread.
More than 10 million pilgrims from over 184 countries visit the Kingdom of Saudi Arabia (KSA) annually to perform religious pilgrimages to Mecca and Medina, either to perform the annual Hajj pilgrimage, which takes place during a certain season, or the Umrah 14, 15. With the emergence of MERS‐CoV on the Arabian Peninsula in 2012, this mass gathering is regarded as a possible transmission scenario with a risk of international spread of the virus 16.
There have been several surveillance studies for MERS‐CoV in Hajj pilgrims; none of which has reported a MERS‐CoV infection 14, 15, 17, 18, 19, 20. No study on returning pilgrims from KSA to the African continent has been conducted, although the Muslim community in Africa consists of over 250 million people, with about one million annual travels to KSA for the pilgrimage 21. We present our findings in screening returning pilgrims at Kotoka International airport in Accra, Ghana, one of West Africa′s major intercontinental air travel hubs.
Materials and methods
Study design
We conducted a cross‐sectional study in November 2013 at the Hajj Village located at the Kotoka International Airport (KIA), Ghana. The Hajj Village is a special arrival destination located on the premises of KIA for all chartered flights from KSA bringing Muslim pilgrims who embarked on the Hajj. Pilgrims were enrolled immediately after their return from the Hajj in KSA. Our goal was to identify the prevalence of MERS‐CoV and the common respiratory viruses human rhinovirus (HRV), respiratory syncytial virus (RSV) and influenza A virus (FLU A).
A standardised questionnaire on demographic and clinical data was completed during a face‐to‐face interview. Both symptomatic and asymptomatic subjects were recruited. Symptomatic subjects were defined as those presenting with any of the following conditions: cough, sore throat, breathing difficulty, runny nose, sneezing or elevated temperature. All symptoms were self‐reported. Asymptomatic subjects did not report any of the symptoms outlined above.
Sampling
Nasopharyngeal specimens were taken with flocked swabs (Copan, Italy) by inserting the swab up the nostril towards the pharynx until resistance was felt. Swabs were then rotated three times to obtain epithelial cells. The swabs were stored in 500 μl RNAlater (Qiagen, Hilden, Germany) and transported to the Kumasi Centre for Collaborative Research in Tropical Medicine (KCCR) laboratory for extraction and testing by real‐time reverse transcription polymerase chain reaction (real‐time RT‐PCR).
Real‐time RT‐PCR analysis
RNA was purified using a Viral RNA Mini Kit (Qiagen) according to manufacturer instructions. All extracts were tested by real‐time RT‐PCR using the Qiagen One‐Step RT‐PCR System (Qiagen) and assays with diagnostic sensitivity for HRV 22, RSV 23, FLU A 24 and MERS‐CoV 25, 26. One‐step real‐time (RT‐) PCR detection was performed on a CFX96 Bio‐Rad real‐time PCR platform (Bio‐Rad, Singapore). In vitro transcribed RNA was used as a positive control. To include only study subjects with a relevant viral load for HRV, RSV and FLU A, only samples with a threshold cycle (CT)‐value below 38 were rated as positive and included in our analysis.
Statistical analysis
Study data were double‐entered into Excel and exported to Stata/SE 12 (Stata Corporation, Texas USA) for analysis. Pearson's chi‐square test was used to analyse categorical variables where appropriate. P‐values ≤ 0.05 were considered significant.
Ethics, consent and approval
Approval for this study was obtained from the Public Health Division of the Ghana Health Service (GHS), Ministry of Health of Ghana and the Port Health Directorate, of the Kotoka International Airport (KIA). All participants were recruited on voluntary basis. The aims and objectives of the study were explained to the pilgrims, and verbal consent was obtained before participants were enrolled.
Results
Pilgrimage cohort
The cohort consisted of 839 adults recruited for the study in November 2013. The mean participant age was 52 years (range 21–85 years). The male‐to‐female ratio was 1:1.2 (Table 1). The pilgrims originated from all 10 geographic regions of Ghana with a majority from the Ashanti region (35.8%), followed by Greater Accra (22.5%) and the Northern Region (20.6%) (Figure 1).
Table 1.
Characteristics of the study population.
| Characteristic | Symptomatic, n (%) | Asymptomatic, n (%) | χ² (P‐value) |
|---|---|---|---|
| Number | 651 (77.6) | 188 (22.4) | |
| Mean age, range | 52, 21–85 | 51, 22–84 | |
| Age groups | |||
| 21–30 | 27 (4.3) | 12 (6.5) | 10.22 (0.116) |
| 31–40 | 112 (17.9) | 40 (21.6) | |
| 41–50 | 131 (21.0) | 41 (22.2) | |
| 51–60 | 187 (29.9) | 40 (21.6) | |
| 61–70 | 133 (21.3) | 37 (20.0) | |
| >71 | 35 (5.6) | 15 (8.1) | |
| Missing | 26 (4.0) | 3 (1.6) | |
| Sex | |||
| Male | 292 (44.9) | 91 (48.4) | 0.74 (0.389) |
| Female | 359 (55.2) | 97 (51.6) | |
| Region of residence | |||
| Ashanti | 226 (34.7) | 74 (39.4) | 8.59 (0.476) |
| Brong Ahafo | 25 (3.8) | 7 (3.7) | |
| Central Region | 19 (2.9) | 3 (1.6) | |
| Eastern Region | 16 (2.5) | 1 (0.5) | |
| Greater Accra | 149 (22.9) | 40 (21.3) | |
| Northern Region | 131 (20.1) | 42 (22.3) | |
| Upper East Region | 20 (3.0) | 5 (2.7) | |
| Upper West Region | 32 (4.9) | 12 (6.4) | |
| Volta | 17 (2.6) | 2 (1.0) | |
| Western Region | 16 (2.5) | 2 (1.0) | |
| Virus detection | |||
| HRV | 114 (17.5) | 27 (14.4) | 1.035 (0.31) |
| RSV | 36 (5.5) | 7 (3.7) | 0.979 (0.32) |
| Flu A | 7 (1.1) | 4 (2.1) | 1.249 (0.26) |
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Figure 1.
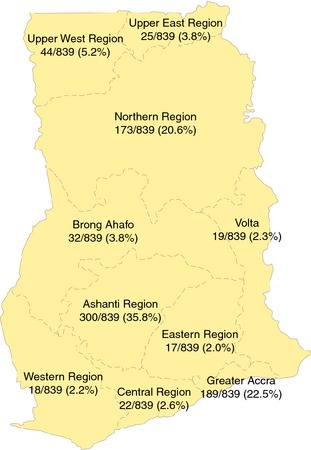
Regional map of Ghana showing the geographical distribution of the Hajj pilgrims.
Virus detection
Overall, 179 (21.3%) of the 839 screened individuals had a positive finding for at least one of the viruses other than MERS. HRV was the most common in 141 (16.8%) individuals, RSV in 43 (5.1%) and FLU A in 11 individuals (1.3%). In 16 (1.9%) pilgrims, more than one virus was detected with 14 (1.7%) RSV/HRV and 2 (0.2%) FLU A/HRV co‐infections).
Clinical presentation
A total 651 (77.6%) of the pilgrims were symptomatic. Both, symptomatic and asymptomatic patients were of comparable age range and mean age (age range, 21–85 years; mean, 52 years and age range, 22–84 years; mean, 51 years, respectively).
The symptomatic returnees presented with cough (593, 91.1%), sore throat (343, 52.7%), elevated temperature (154, 23.7%), runny nose or sneezing (152, 23.3%), and breathing difficulty (124, 19.0%).
A total 146 (22.4%) of the symptomatic returnees tested positive for at least one respiratory virus compared with 33 (17.6%) of the asymptomatic pilgrims who had at least one detectable virus in their sample (χ² = 2.06, P < 0.001).
Of the symptomatic pilgrims, 114 (17.5%) tested positive for HRV; 36 (5.5%) and 7 (1.1%) tested positive for RSV and FLU A, respectively. Twenty‐seven (14.4%) of the asymptomatic returnees tested positive for HRV, 7 (3.7%) for RSV and 4 (2.1%) were positive for FLU A. Differences between the individual virus and the two groups of pilgrims was not significant (Table 1).
Of the 593 pilgrims who presented with cough, 135 (22.8%) tested positive for at least one virus. Seventy‐six (22.2%) of those who presented with sore throat also tested positive for at least one virus, while 33 (21.4%) of those who presented with elevated temperature also had a respiratory virus. Of those who presented with runny nose and breathing difficulty, 27 (17.8%) and 32 (25.8%) tested positive for at least one virus, respectively.
Discussion
Several surveillance studies on MERS‐CoV in pilgrims returning from the annual Hajj have been performed after the emergence of MERS‐CoV. In none of these studies, conducted during the last three Hajj seasons in 2012, 2013 and 2014, was MERS‐CoV detected 14, 15, 17, 18, 19, 20, 27.
The first study after the emergence of MERS‐CoV investigated 154 French Hajj pilgrims participating in the 2012 Hajj prior to returning to their home country for the presence of MERS‐CoV in nasal swabs 18. No MERS‐CoV infection was detected, but a high rate of respiratory symptoms was reported by 83.4% of pilgrims, with a subset of 41.0% fulfilling the criteria for influenza‐like illness (ILI), which is defined by cough, sore throat and fever. However, this study did not test for viruses other than MERS‐CoV. A second study from France assessed the rate of positive viral findings including MERS‐CoV in a cohort of pilgrims before (n = 165), during (n = 70), and at the end (prior to the departure to their home country; n = 154) of their Hajj pilgrimage in the 2012 season 17, without detection of MERS‐CoV in any of the study subjects. The largest study on MERS‐CoV prevalence in Hajj pilgrims so far was performed during the Hajj season of 2013 with a total of 5235 adult pilgrims from 22 countries, all sampled in KSA upon arrival (3210 pilgrims) or departure from the Hajj (2025). No MERS‐CoV positive pilgrim was identified in this study; however, no other causes of viral illness have been assessed in this cohort 14.
The role of respiratory infections during the Hajj is nevertheless of importance, independent of the emergence of MERS‐CoV: respiratory illness was the leading cause of hospital admissions at the Hajj 28. A review on available cross‐sectional studies revealed an estimated 20% to 80% of upper respiratory tract infection among Hajj pilgrims, with influenza and rhinoviruses as the most common viral agent 29. This is in line with findings from the above‐mentioned studies on MERS‐CoV in Hajj pilgrims: in the study by Benkouiten et al. 17 , the most commonly detected virus before, during and at the end of the Hajj was HRV in 5 of 165 (3.0%), 19 of 70 (27.1%) and 13 of 154 (8.4%), respectively. A significantly higher number of viral infections during and at the end of the Hajj were seen with 27 of 70 (38.6%) individuals positive for at least one respiratory virus during the Hajj and 17 of 154 (11.0%) pilgrims positive at the end of the Hajj compared to 8 of 165 (4.8%) pilgrims who were positive before their departure from France to KSA. 17. This suggests a probable rapid acquisition of respiratory viruses among pilgrims during their stay. Benkouiten et al. 17 report the detection of FLU A during the Hajj in 6 of 70 (8.6%) pilgrims, but did not detect FLU A at the end of the Hajj. In contrast, we found 1.3% of pilgrims positive for FLU A upon return to Ghana. In contrast to our data, Benkouiten reported only a few RSV infections with a positive finding for RSV in only 1 of 70 (1.4%) pilgrim during the Hajj. Despite the slightly different sampling time point (upon departure in KSA and after return to Ghana), further reasons for the differences between studies may be the small number reported in the cohorts, with only 165, 70 and 154 pilgrims in the French study 17 versus our cohort of 839 individuals. The different ethnic and geographic background of the cohort and therefore a different pre‐existing immunity may also account for the observed differences. There may also have been a difference between the seasons (2012 Hajj season versus 2013 Hajj season). Notably, in a follow‐up study performed during the 2013 Hajj season, the same group reported a higher prevalence of influenza than previously found, with 10 of 129 pilgrims testing positive for influenza viral RNA (8 influenza A (H3N2), 1 for influenza A (H1N1), 1 for influenza B). Furthermore, a high rate of respiratory symptoms was found with 117 of 129 (90.7%) of pilgrims reporting respiratory symptoms while still in KSA and 55 of 129 (78.6%) in the 3–5 weeks after their return 19, but no other respiratory viruses were screened for in the study.
Interestingly, a study assessing the aetiology of severe community‐acquired pneumonia in returning Hajj pilgrims showed 21 of 26 (80.7%) pilgrims in whom a respiratory pathogen (viruses or bacteria) was detected were positive for a viral finding 14, 15. Similar to our cohort, the most common respiratory virus was HRV, detected in 57.7% of positive samples, followed by FLU A in 23.1%. MERS‐CoV was not the cause of severe CAP in any of the hospitalised pilgrims investigated.
Our data support the findings from earlier studies on lack of MERS‐CoV infections in returning pilgrims during the 2013 Hajj season. However, in the light of the rapidly increasing number of MERS‐CoV cases in early 2014 (more than 800 documented cases thus far) and the upcoming 2015 Hajj season, further surveillance is necessary to confirm the absence of MERS‐CoV transmission in the 2015 pilgrimage cohort. This is of utmost importance considering the severe overcrowding situations during these mass gatherings. In developing countries with limited resources for molecular diagnostics and a diverse spectrum of febrile aetiologies, early detection of MERS‐CoV‐infected patients will remain a challenge.
Acknowledgements
We thank all pilgrims for their willingness to participate in this study. The work was funded by the KCCR and the German Research Foundation.
References
- 1. Woo PC, Lau SK, Huang Y, Yuen KY. Coronavirus diversity, phylogeny and interspecies jumping. Exp Biol Med (Maywood) 2009: 234: 1117–1127. [DOI] [PubMed] [Google Scholar]
- 2. Drosten C, Gunther S, Preiser W et al Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med 2003: 348: 1967–1976. [DOI] [PubMed] [Google Scholar]
- 3. de Groot R, Baker SC, Baric RS et al Middle East respiratory syndrome coronavirus (MERS‐CoV): announcement of the Coronavirus Study Group. J Virol 2013: 87: 7790–7792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Drosten C, Muth D, Corman VM et al An observational, laboratory‐based study of outbreaks of MERS‐Coronavirus in Jeddah and Riyadh, Kingdom of Saudi Arabia, 2014. Clin Infect Dis 2014: 60: 369–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zaki AM, van Boheemen S, Bestebroer TM, Osterhaus AD, Fouchier RA. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med 2012: 367: 1814–1820. [DOI] [PubMed] [Google Scholar]
- 6. Bermingham A, Chand MA, Brown CS et al Severe respiratory illness caused by a novel coronavirus, in a patient transferred to the United Kingdom from the Middle East, September 2012. Euro Surveill 2012: 17: 20290. [PubMed] [Google Scholar]
- 7. Bialek SR, Allen D, Alvarado‐Ramy F et al First confirmed cases of Middle East respiratory syndrome coronavirus (MERS‐CoV) infection in the United States, updated information on the epidemiology of MERS‐CoV infection, and guidance for the public, clinicians, and public health authorities ‐ May 2014. MMWR Morb Mortal Wkly Rep 2014: 63: 431–436. [PMC free article] [PubMed] [Google Scholar]
- 8. Drosten C, Seilmaier M, Corman VM et al Clinical features and virological analysis of a case of Middle East respiratory syndrome coronavirus infection. Lancet Infect Dis 2013: 13: 745–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kraaij‐Dirkzwager M, Timen A, Dirksen K et al “Middle East respiratory syndrome coronavirus (MERS‐CoV) infections in two returning travellers in the Netherlands, May 2014. Euro Surveill 2014: 19: 20817. [DOI] [PubMed] [Google Scholar]
- 10. Mailles A, Blanckaert K, Chaud P et al First cases of Middle East Respiratory Syndrome Coronavirus (MERS‐CoV) infections in France, investigations and implications for the prevention of human‐to‐human transmission, France, May 2013. Euro Surveill 2013: 18 8: pii=20502. [PubMed] [Google Scholar]
- 11. Premila Devi J, Noraini W, Norhayati R et al Laboratory‐confirmed case of Middle East respiratory syndrome coronavirus (MERS‐CoV) infection in Malaysia: preparedness and response, April 2014. Euro Surveill 2014: 19: 20797. [DOI] [PubMed] [Google Scholar]
- 12. Puzelli S, Azzi A, Santini MG et al Investigation of an imported case of Middle East Respiratory Syndrome Coronavirus (MERS‐CoV) infection in Florence, Italy, May to June 2013. Euro Surveill 2013: 18: pii=20564. [DOI] [PubMed] [Google Scholar]
- 13. Tsiodras S, Baka A, Mentis A et al A case of imported Middle East Respiratory Syndrome coronavirus infection and public health response, Greece, April 2014. Euro Surveill 2014: 19: 20782. [DOI] [PubMed] [Google Scholar]
- 14. Memish ZA, Almasri M, Turkestani A, Al‐Shangiti AM, Yezli S. Etiology of severe community‐acquired pneumonia during the 2013 Hajj‐part of the MERS‐CoV surveillance program. Int J Infect Dis 2014: 25: 186–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Memish ZA, Assiri A, Almasri M et al Prevalence of MERS‐CoV nasal carriage and compliance with the Saudi health recommendations among pilgrims attending the 2013 Hajj. J Infect Dis 2014b: 210: 1067–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Khan K, Sears J, Hu VW et al Potential for the international spread of Middle East respiratory syndrome in association with mass gatherings in Saudi Arabia. PLoS Curr 2013: 5: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Benkouiten S, Charrel R, Belhouchat K et al Circulation of respiratory viruses among pilgrims during the 2012 Hajj pilgrimage. Clin Infect Dis 2013: 57: 992–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gautret P, Charrel R, Belhouchat K et al Lack of nasal carriage of novel corona virus (HCoV‐EMC) in French Hajj pilgrims returning from the Hajj 2012, despite a high rate of respiratory symptoms. Clin Microbiol Infect 2013: 19: E315–E317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gautret P, Charrel R, Benkouiten S et al Lack of MERS coronavirus but prevalence of influenza virus in French pilgrims after 2013 Hajj. Emerg Infect Dis 2014: 20: 728–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rashid H, Barasheed O, Booy R. Acute febrile respiratory infection symptoms in Australian Hajjis at risk of exposure to Middle East respiratory syndrome coronavirus. Med J Aust 2013: 199: 453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zumla A, Mwaba P, Bates M, Al‐Tawfiq JA, Maeurer M, Memish ZA. The Hajj pilgrimage and surveillance for Middle East Respiratory syndrome coronavirus in pilgrims from African countries. Tropical Med Int Health 2014: 19: 838–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lu X, Holloway B, Dare RK et al Real‐time reverse transcription‐PCR assay for comprehensive detection of human rhinoviruses. J Clin Microbiol 2008: 46: 533–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kuypers J, Wright N, Morrow R. Evaluation of quantitative and type‐specific real‐time RT‐PCR assays for detection of respiratory syncytial virus in respiratory specimens from children. J Clin Virol 2004: 31: 123–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Spackman E, Senne DA, Myers T et al Development of a real‐time reverse transcriptase PCR assay for type A influenza virus and the avian H5 and H7 hemagglutinin subtypes. J Clin Microbiol 2002: 40: 3256–3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Corman VM, Eckerle I, Bleicker T et al Detection of a novel human coronavirus by real‐time reverse‐transcription polymerase chain reaction. Euro Surveill 2012: 17: pii/20288. [DOI] [PubMed] [Google Scholar]
- 26. Corman VM, Muller MA, Costabel U et al Assays for laboratory confirmation of novel human coronavirus (hCoV‐EMC) infections. Euro Surveill 2012: 17: pii: 20334. [DOI] [PubMed] [Google Scholar]
- 27. Aberle JH, Popow‐Kraupp T, Kreidl P, Laferl H, Heinz FX & Aberle SW (2014). Influenza A and B viruses but not MERS‐CoV in Hajj Pilgrims, Austria, 2014. Emerg Infect Dis (ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Al‐Ghamdi SM, Akbar HO, Qari YA, Fathaldin OA, Al‐Rashed RS. Pattern of admission to hospitals during muslim pilgrimage (Hajj). Saudi Med J 2003: 24: 1073–1076. [PubMed] [Google Scholar]
- 29. Al‐Tawfiq JA, Zumla A, Memish ZA. Travel implications of emerging coronaviruses: SARS and MERS‐CoV. Travel Med Infect Dis 2014: 12: 422–428. [DOI] [PMC free article] [PubMed] [Google Scholar]


