Figure 2.
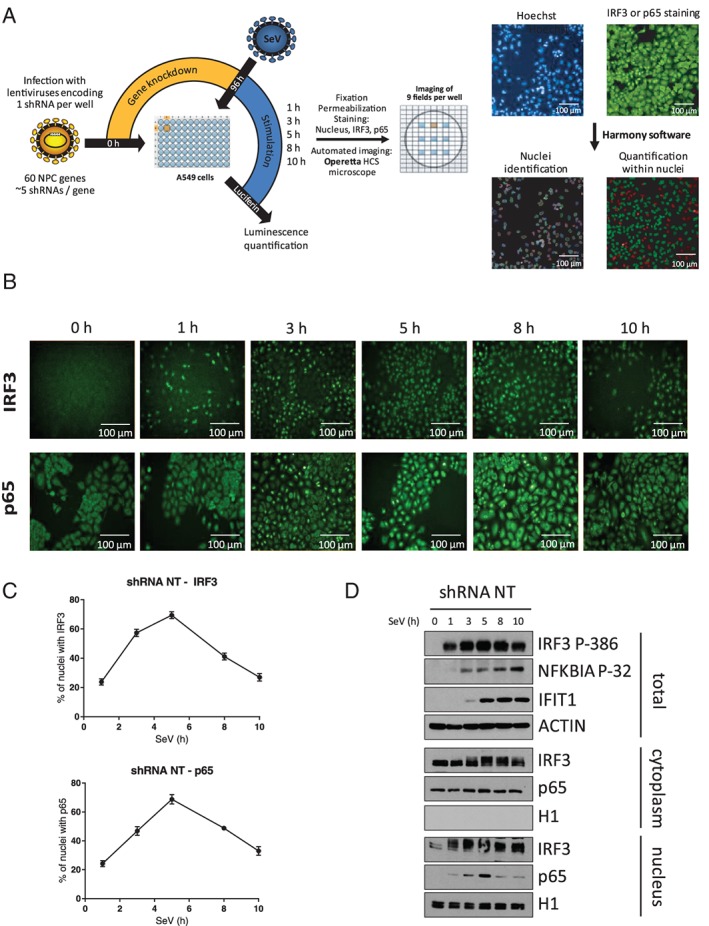
Microscopy‐based High Content Screening (HCS) of IFN regulatory factor 3 (IRF3) and NF‐κB p65 nuclear translocation. A, Overview of the microscopy‐based gene silencing screen. A549 cells plated in 96‐well plates are transduced with 5 independent lentivirus‐encoding short hairpin RNA (shRNA) per gene (1 shRNA per well) at a multiplicity of infection (MOI) of 10 for 4 days to silence expression of 60 nuclear transport factors. A control shRNA NT is included in each 96‐well plate. Cells are infected with Sendai virus (SeV) for 1, 3, 5, 8 or 10 hours before fixation, permeabilization, Hoechst nuclear labeling and antibody staining of IRF3 or NF‐κB p65 with Alexa Fluor 488 (green). Images of cells are captured in 9 pre‐determined fields for each well using an Operetta HCS Microscope. Images are processed using Harmony software to delimitate the nuclear region and measure the fluorescence intensity of IRF3 or NF‐κB p65 within the nucleus. For each 96‐well plate, a fluorescence cut‐off is set to allow automated discrimination of cells with (green) or without (red) IRF3 or NF‐κB p65 nuclear staining and to calculate the percentage of cells for each shRNA‐mediated gene knockdown. Scale bar is equal to 100 μM. B, Representative time course imaging performed with the control shRNA NT showing the nuclear translocation of IRF3 or NF‐κB p65 over a 10‐hour Sendai virus (SeV) infection (1 representative of 9 field images). Scale bar is equal to 100 μM. C, Graphic representation of the microscopy image‐based analysis showing an increase in the percentage of cells with positive nuclear staining for IRF3 or NF‐κB p65 culminating with ~75% of positive cells at 5 hours post‐infection followed by a decrease to ~30% of positive cells at 10 hours. D, Immunoblot analysis of total cell lysates, cytoplasmic and nuclear extracts of A549 cells infected with lentivirus‐encoding shRNA NT at a MOI of 10 for 3 days and infected with SeV for 0, 1, 3, 5, 8 and 10 hours prior to cell harvesting
