Figure 2.
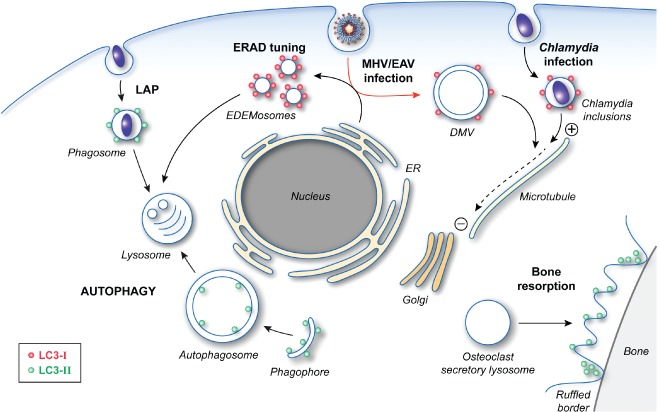
The cellular functions of LC3. After post‐translational processing by ATG4, LC3‐I is covalently linked to PE to generate LC3‐II through the action of two ubiquitin‐like conjugation systems. The formation of lipidated LC3 is essential for autophagosome formation by mediating the expansion of the phagophore. LC3‐II is also involved in LAP as well as the specialized secretion by osteoclasts, which is essential for the establishment of the ruffled borders for bone resorption. The role of LC3‐II in these two latter pathways is unclear, but its localization and function do not require an intact ATG machinery. Precursor LC3‐I is also carrying out cellular functions, which do not appear to involve most of the other ATG proteins. LC3‐I is recruited together with MAP1 proteins onto Chlamydia inclusions and allows the redistribution of these bacteria‐containing compartments in proximity of the Golgi through its interaction with the microtubules. LC3‐I is also associated with the surface of the EDEMosomes via its interaction with the protein cargo receptor Sel1L. The role of LC3‐I in the ERAD tuning pathway remains unknown, but it could act as an adaptor for either a protein vesicle coat or the cytoskeleton network. Viruses, such as MHV and EAV, hijack this transport route to generate their replicative DMVs, which are also decorated with LC3‐I (red arrow). LC3‐I is essential for both the ERAD tuning and the replication of these viruses. In this latter context, LC3‐I could mediate the microtubule‐dependent subcellular redistribution of DMVs.
