Table 1.
In vitro antiproliferative activities of 2–(4-((-pyrimidin-2-yl)amino)benzoyl) hydrazine-1-carboxamide derivatives (Series A) on two selected cancer cell lines.a
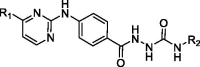 | |||||
|---|---|---|---|---|---|
| Compounds | R1 | R2 | X | IC50[μM] |
|
| HepG2 | A549 | ||||
| 6a | 4-Pyridyl | n-Butyl | O | 5.32 ± 0.07 | 21.69 ± 1.33 |
| 6b | 4-Pyridyl | t- Butyl | O | 5.39 ± 0.11 | 18.09 ± 1.25 |
| 6c | 4-Pyridyl | Cyclohexyl | O | 2.17 ± 0.08 | 8.39 ± 0.92 |
| 6d | 4-Pyridyl | Cyclopentyl | O | 2.71 ± 0.13 | 16.26 ± 1.21 |
| 6e | 4-Pyridyl | p-Tolyl | O | 1.28 ± 0.09 | 11.27 ± 1.05 |
| 6f | 4-Pyridyl | 2,4-difluorophenyl | O | 8.10 ± 0.12 | 26.38 ± 1.42 |
| 6g | 4-Pyridyl | 3-chloro-4-methylphenyl | O | >40 | >40 |
| 6h | 4-Pyridyl | Phenethyl | O | 3.89 ± 0.04 | 13.11 ± 1.11 |
| 6i | 3-Pyridyl | n-Butyl | O | >40 | 38.93 ± 1.59 |
| 6j | 3-Pyridyl | Cyclopentyl | O | 5.73 ± 0.26 | 17.09 ± 1.23 |
| 6k | 3-Pyridyl | p-Tolyl | O | >40 | 8.385 ± 0.92 |
| 6l | 3-Pyridyl | Phenethyl | O | 5.48 ± 0.21 | 23.71 ± 1.37 |
| 6m | 2-Pryidyl | n- Butyl | O | >40 | >40 |
| 6n | 2-Pryidyl | t-Butyl | O | 15.71 ± 0.11 | >40 |
| 6o | 2-Pryidyl | Cyclopentyl | O | >40 | >40 |
| 6p | 2-Pryidyl | m-Tolyl | O | 23.31 ± 0.07 | >40 |
| 6q | 2-Pryidyl | 3,5-dimethylphenyl | O | 20.32 ± 0.14 | >40 |
| 6r | 4-Chlorophenyl | t- Butyl | O | 4.48 ± 0.09 | 19.49 ± 1.29 |
| 6s | 4-Chlorophenyl | Ethyl | O | 20.34 ± 0.34 | >40 |
| 6t | 4-Chlorophenyl | Cyclohexyl | O | 13.08 ± 0.46 | >40 |
| 6A | 4-Pyridyl | Phenyl | S | 5.13 ± 0.14 | 5.09 ± 0.70 |
| 6B | 4-Pyridyl | Benzyl | S | 0.61 ± 0.07 | 15.95 ± 1.20 |
| 6C | 3-Pyridyl | Benzyl | S | 1.47 ± 0.42 | 17.57 ± 1.24 |
| 6D | 2-Pryidyl | Phenyl | S | 6.74 ± 0.05 | 38.51 ± 1.58 |
| 6E | 2-Pryidyl | Benzyl | S | 4.59 ± 0.11 | >40 |
| 6F | Phenyl | Cyclohexyl | S | >40 | 30.79 ± 1.48 |
| 6G | Phenyl | Phenyl | S | >40 | 20.68 ± 1.31 |
| 6H | Phenyl | Benzyl | S | >40 | 22.27 ± 1.34 |
| 6I | 4-Chlorophenyl | Phenyl | S | 17.24 ± 0.07 | 28.32 ± 1.45 |
| 6J | 4-Chlorophenyl | Benzyl | S | 1.29 ± 0.09 | 12.76 ± 1.10 |
| Sorafenib | 16.89 ± 1.22 | >40 | |||
aData are means ± SD of triplicate experiments.
