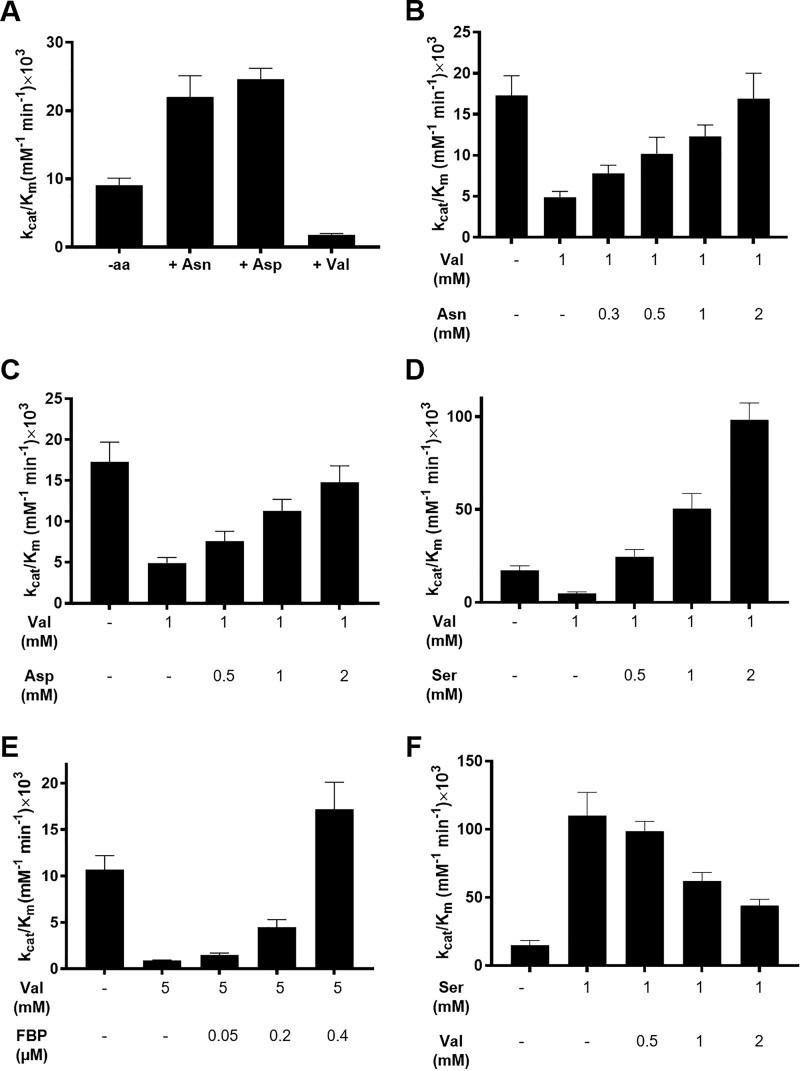
Figure 1.
Effect of Asn, Asp, and Val on PKM2 activity and activation of Val inhibited PKM2 by various effector molecules. The activity assays with Asn, Asp, and Val were performed with 20 nm PKM2 with the PEP concentration varying between 0.1 and 12 mm. For the reactivation assays, the enzyme concentration was 12.5 nm, and the PEP concentration range was 0.1–20 mm. The ADP concentration was kept fixed at 0.8 mm for all experiments. A, varying concentrations of AAs were incubated with PKM2, and kinetic data at 450 μm (Asp and Asn) and 4 mm (Val) concentration were chosen to calculate the catalytic efficiency. The kcat/Km increases from 8.2 ± 0.8 × 103 to 22 ± 3.1 × 103 mm−1 min−1 in the presence of Asn and from 10.5 ± 2.4 × 103 to 24.6 ± 1.6 × 103 mm−1 min−1 with Asp. Val decreases the catalytic efficiency from 8.6 ± 1.4 × 103 to 1.8 ± 0.2 × 103 mm−1 min−1. B–E, PKM2 was inhibited with Val (1 or 5 mm), and the activity was resuscitated by adding an increasing amount of Asn, Asp, Ser, and FBP. Kinetic parameters are listed in Table S2. F, displacement of Ser activated PKM2 with increasing concentrations of Val. The catalytic efficiency decreases from 110 ± 17 × 103 mm−1 min−1 in the presence of 1 mm Ser to 44 ± 4.6 × 103 mm−1 min−1 in the presence of 1 mm Ser and 2 mm Val. Kinetic parameters are listed in Table S3.