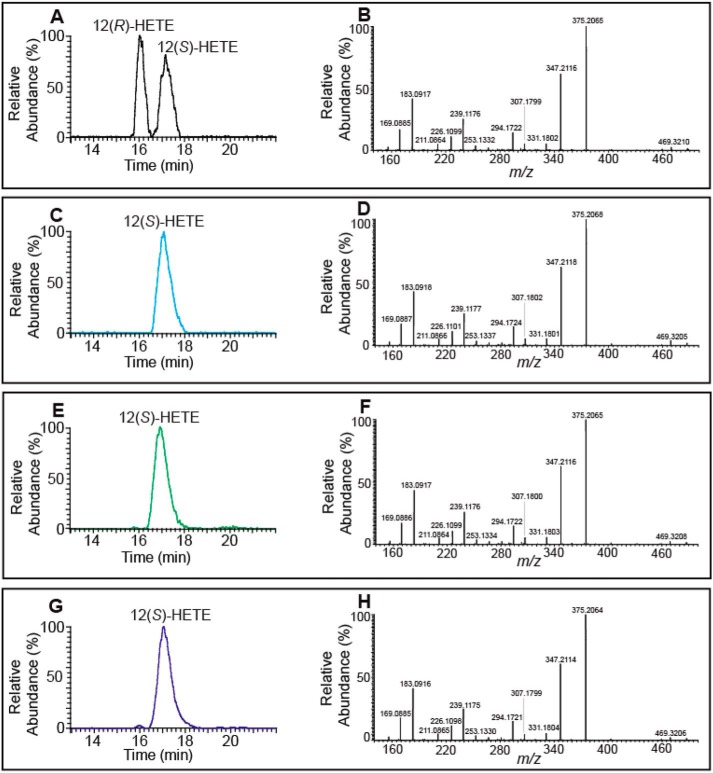
Figure 3.
Chiral-phase HPLC-MS analyses of the 12-HETE-AMPP derivatives generated by 12-LOX–catalyzed oxidation of 2-AA-LPC and 2-AA-LPE. 12-LOX generated hydroperoxy eicosanoid-lysophospholipids were treated with cPLA2α and then reduced by triphenylphosphine. Next, the resultant eicosanoid products were derivatized with AMPP and analyzed by chiral-phase HPLC-MS. A, chromatogram of standard racemic AMPP-derivatized 12(R/S)-HETE. B, MS/MS product ion spectrum of standard reference racemic AMPP-derivatized 12(R/S)-HETE. C, chromatogram of standard AMPP-derivatized 12(S)-HETE. D, MS/MS product ion spectrum of standard 12(S)-HETE; E, chromatogram of the AMPP-derivatized eicosanoid(s) originating from the 12-LOX oxidation products of 2-AA-LPC. F, MS/MS product ion spectrum of the AMPP-derivatized eicosanoid(s) originating from the 12-LOX oxidation products of 2-AA-LPC. G, chromatogram of the AMPP-derivatized eicosanoid(s) originating from the 12-LOX oxidation products of 2-AA-LPE. H, MS/MS product ion spectrum of the AMPP-derivatized eicosanoid(s) originating from the 12-LOX oxidation products of 2-AA-LPE.