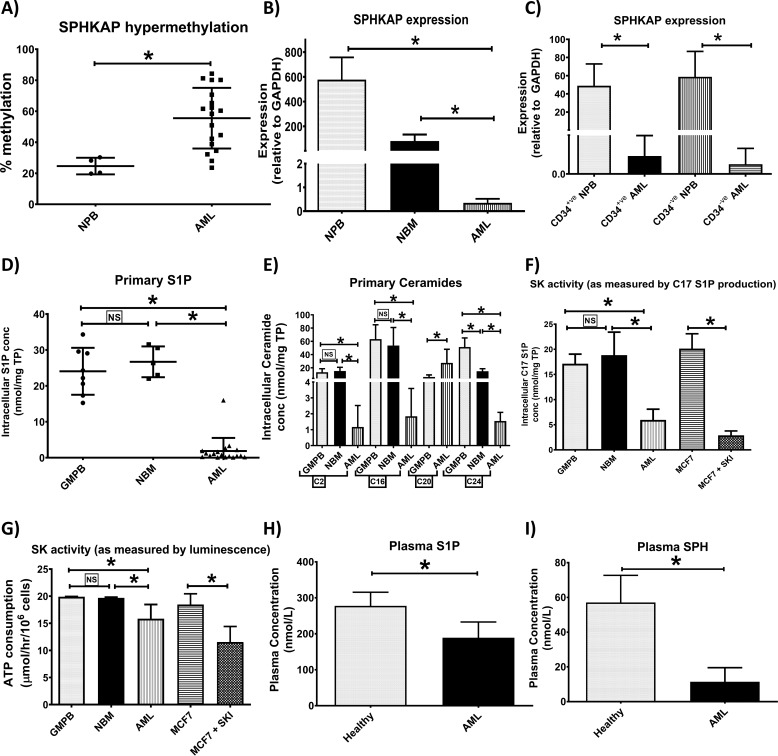
Figure 1.
S1P and ceramides are down-regulated in AML due to SK hypofunction. Using bisulfite pyrosequencing, SPHKAP (the gene that produces SKIP) hypermethylation was confirmed in primary AML (n = 18) compared with NPB (n = 4) samples (A). SKIP underexpression was confirmed in blood samples from patients with AML (n = 18) compared with healthy volunteer NPB (n = 4) and normal bone marrow samples (NBM, n = 5) as studied by qPCR (B). Reduced SKIP expression involved both CD34+ and CD34− components of AML primary samples (n = 4) compared with NBP (n = 4) (C) as studied by qPCR. Scatter plots show lower S1P (D) and ceramide (E) concentrations in primary AML cells (n = 18) versus NBM (n = 5) and G-mobilized peripheral blood cells (GMPB) (n = 8). Bar charts show lower SK function as measured by UPLC-MS/MS detection of C17 S1P production (F) after 24 h incubation with 1 μm C17 sphingosine substrate in primary AML cells (n = 6) versus NBM (n = 5) and GMPB (n = 6) MCF7 cell line was used as positive control and 10 μm SKI 5C was used to inhibit SK activity. Lower SK function in primary AML cells (n = 18) versus NBM (n = 3) and GMPB (n = 3) was confirmed using another method for measuring SK activity depending on ELISA detection of ATP consumption due to SK enzymatic activity (G). Cell lysate from the MCF7 cell line was used as source for SK enzyme (positive control) and 10 μm SKI 5C was used to inhibit SK activity. Plasma S1P (H) and sphingosine (I) concentrations were lower in AML patients (n = 15) versus healthy volunteers (n = 5) as measured by UPLC-MS/MS. * = p < 0.05; NS, not significant (p > 0.05) as measured by t test.