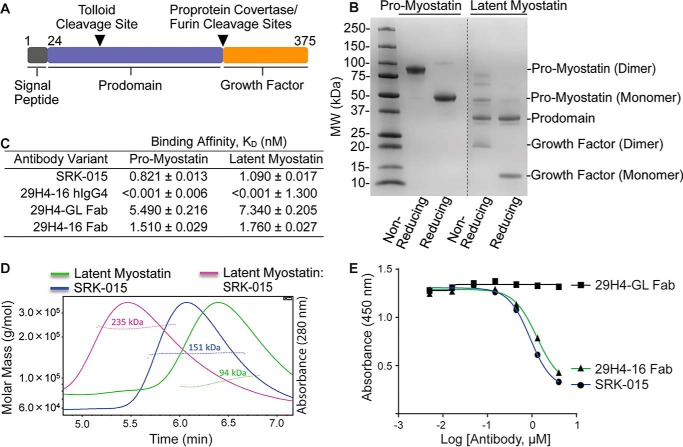
Figure 1.
SRK-015 and analogs bind and block proteolytic activation of myostatin. A, linear cartoon of pro-myostatin showing the signal peptide (black), prodomain (blue), and growth factor (orange). The tolloid and furin protease cleavage sites are indicated by inverted triangles. B, profile of Expi293F-expressed and purified pro- and latent myostatin run under nonreducing and reducing SDS-PAGE. MW, molecular weight. C, relative binding affinities of SRK-015 and analogs to pro- and latent myostatin, determined using biolayer interferometry (FortéBio Octet). Data are presented as mean ± S.E. of duplicate measurements. D, SEC-MALS profiles of latent myostatin, SRK-015, and the latent myostatin–SRK-015 complex. The dotted lines across the peaks represent the molar mass of the samples determined from the light scattering data. E, ELISA-based myostatin activity assay showing that SRK-015 and analogs block proteolytic activation of myostatin. The data are representative of three independent experiments.