Figure 3.
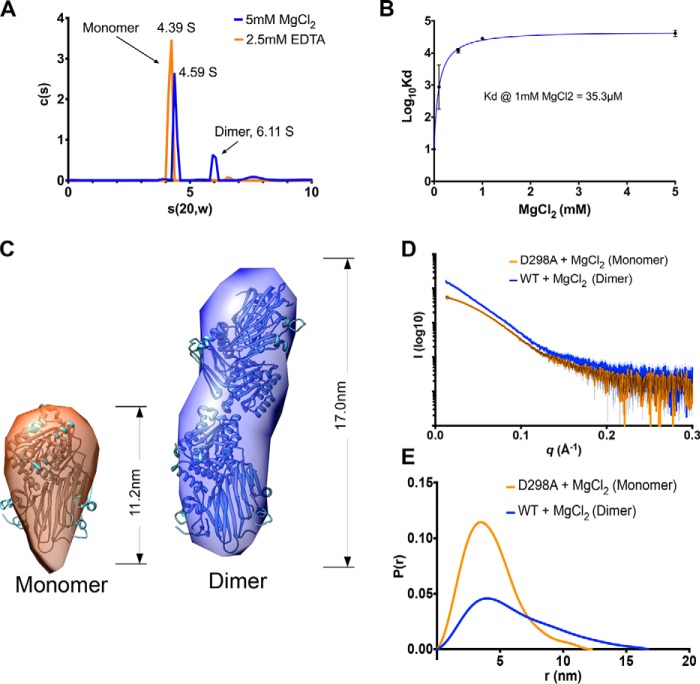
HC1 forms metal ion-dependent dimers. A, a plot of sedimentation coefficient distributions (c(s)) versus s(apparent)) for WT rHC1 derived from velocity AUC analysis. In the presence of 2.5 mm EDTA (orange) 93% of the rHC1 protein is in a monomeric state (s(20,w) = 4.39 S), and there is no detectable dimer present. In 5 mm MgCl2 (blue), 64% of the protein is monomeric (s(20,w) = 4.59 S), and 21% of material is dimeric (s(20,w) = 6.11 S). B, plot of Log10KD versus MgCl2 concentration, derived from equilibrium AUC measurements. At 0 mm MgCl2 (achieved by conducting the experiment in 2.5 mm EDTA), no dimerization was detected. Maximal binding affinity (for self-association of the rHC1 dimer) was reached at ∼1 mm MgCl2, i.e. close to the concentration of free Mg2+ ions in plasma. C, ab initio SAXS models for the HC1 monomer (left panel) and dimer (right panel) where the HC1 structure has been modeled into the SAXS envelopes. D and E, buffer-subtracted SAXS scattering curves for HC1 D298 monomer (orange) and WT dimer (blue) (D) and their derived P(r) versus distance plots (E), consistent with WT HC1 forming an elongated Mg2+-dependent dimer and the MIDAS site mutant (D298A) being monomeric.