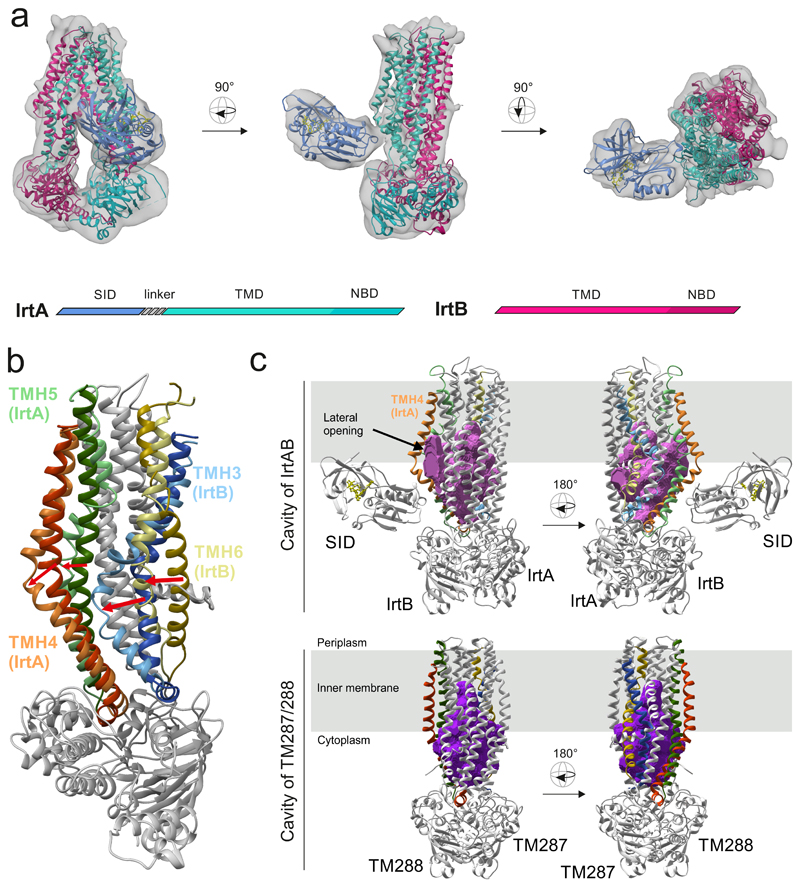
Figure 1. Structure of IrtAB.
a, X-ray structures of IrtAB devoid of the SID (2.7 Å, IrtA in turquoise and IrtB in purple) and the isolated SID (1.8 Å, blue with the bound FAD molecule shown in yellow as sticks) were fitted into the cryo-EM map (6.9 Å) of full-length IrtAB. The domain organization of the IrtAB heterodimer is shown at the bottom. b, Superimposition of IrtB plus domain-swap-helices TMH4 and 5 of IrtA with TM288 plus domain-swap-helices TMH4 and 5 of TM287. TMH6 (IrtB/TM288), TMH3 (IrtB/TM288), TMH4 (IrtA/TM287) and TMH5 (IrtA/TM287) are colored as indicated and the rest is shown in grey. Arrows indicate the structural changes from TM287/288 (darker colors) to IrtAB (lighter colors). c, Inward-facing cavities of IrtAB and TM287/288 (PDB: 4Q4A). TMHs with strong differences between IrtAB and TM287/288 are colored as in (b). The inward-facing cavity of IrtAB is collapsed at the upper third as compared to TM287/288 mainly due to the movement of TMH6 of IrtB. The bulged-out domain swap helices TMH4 and TMH5 of IrtA result in a lateral access to the cavity from the inner leaflet of the lipid bilayer (indicated in grey).