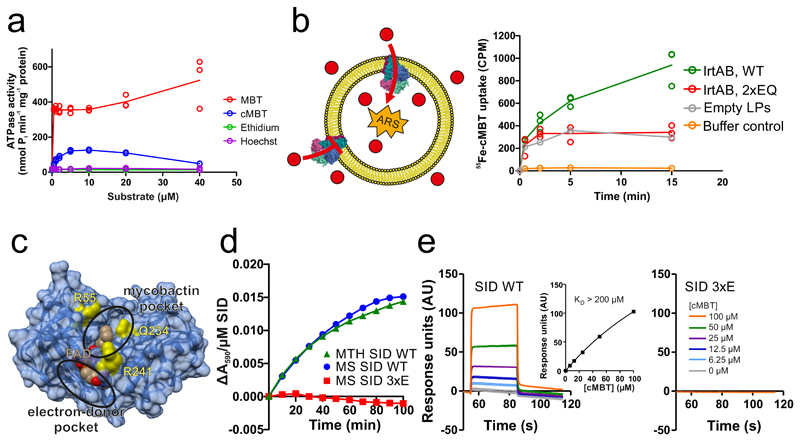
Figure 2. Biochemical characterization of IrtAB.
a, ATPase activity of IrtAB reconstituted into nanodiscs in the presence of increasing concentrations of Fe-MBT, Fe-cMBT, Ethidium and Hoechst. Data points are technical triplicates and curves cross mean values. b, In vitro transport of Fe-cMBT mediated by wildtype (WT) and inactive (2xEQ mutant) IrtAB reconstituted into proteoliposomes. An ATP regenerating system (ARS) was incorporated into the vesicle lumen (left panel). Uptake of radioactively labeled 55Fe-cMBT into the vesicle lumen was measured over time (right panel). As a control, the assay was performed with empty liposomes (LPs) or buffer only. For WT and 2xEQ mutant, data points are technical triplicates and curves cross mean values. c, Structure of the SID with the electron-donor and mycobactin pockets highlighted. FAD is shown as spheres and colored in beige. To generate the 3xE mutant, three conserved residues lining the mycobactin pocket (yellow) were mutated to glutamate. d, Reduction of Fe(III)-cMBT by M. thermoresistibile (MTH) and M. smegmatis (MS) SIDs using NADPH as electron donor and Ferene as a reporter probe of released Fe(II) (Amax = 590 nm). The 3xE mutant served as negative control. Representative data of biological duplicates are shown. e, Binding of Fe-cMBT to the M. smegmatis SID as measured by SPR using Fe-cMBT concentrations as indicated in the right panel. SPR sensorgrams were recorded for the wildtype SID (left panel) or the 3xE mutant (right panel). Fitting of equilibrium binding values indicated a KD > 200 μM (left panel, inset). The SPR experiment was performed once.