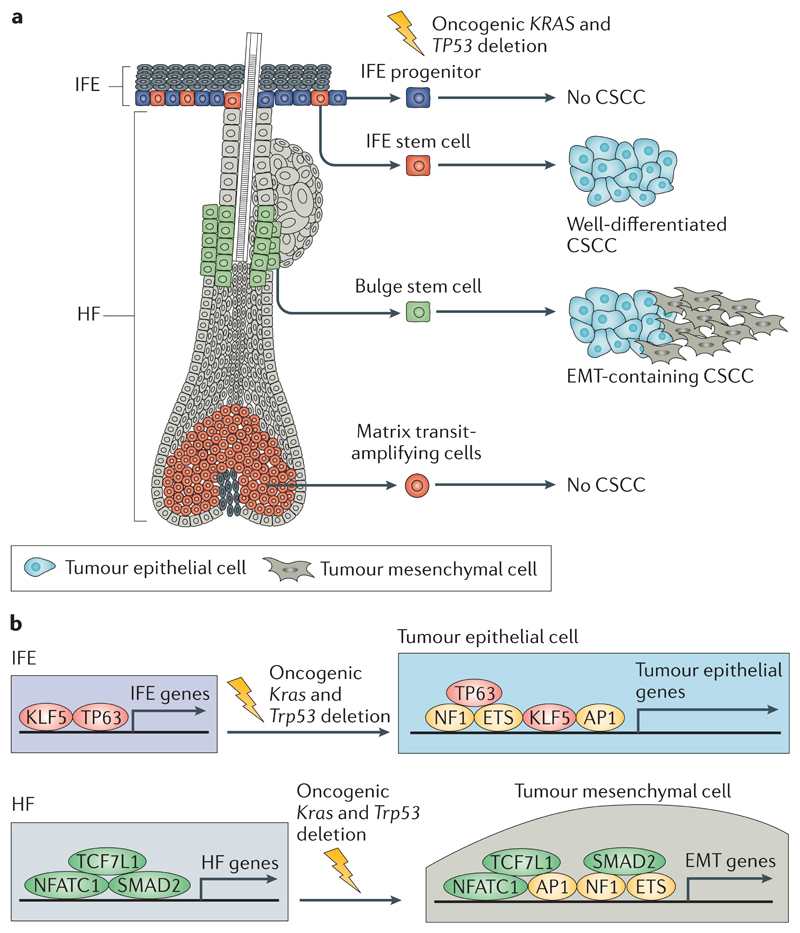
Fig 4. The cells at the origin of CSCC.
a | Activation of oncogenic Kras and deletion of Trp53 in interfollicular epidermis (IFE) and hair follicle (HF) stem cells lead to cutaneous squamous cell carcinoma (CSCC) formation, whereas these gene alterations in IFE progenitors or matrix transit-amplifying cells do not lead to CSCC formation. Oncogenic activation of Kras and deletion of Trp53 in IFE stem cells lead to the generation of well-differentiated CSCCs, whereas activation of the same oncogenic hits in HF stem cells leads to the generation of CSCCs with epithelial to mesenchymal transition (EMT) features. b | The transcriptional and epigenetic landscape of the cell of origin influences tumour differentiation. Upon oncogenic Kras expression and Trp53 deletion, a core of transcription factors (including members of the adaptor protein 1 (AP1), E26 transformation-specific (ETS) and nuclear factor 1 (NF1) families) promote tumour gene expression independently of the cell of origin. In addition to this core of transcription factors, lineage-specific transcription factors controlled by the cells of origin of CSCCs influence the specific differentiation of the tumours. Tumour protein 63 (TP63) and Krüppel-like factor 5 (KLF5) promote the expression of IFE genes and the development of well-differentiated squamous cell carcinomas (SCCs), whereas SMAD family member 2 (SMAD2), nuclear factor of activated T cell, cytoplasmic 1 (NFATC1) and transcription factor 7-like 1 (TCF7L1) promote the expression of HF genes and the development of SCCs in which EMT occurs.