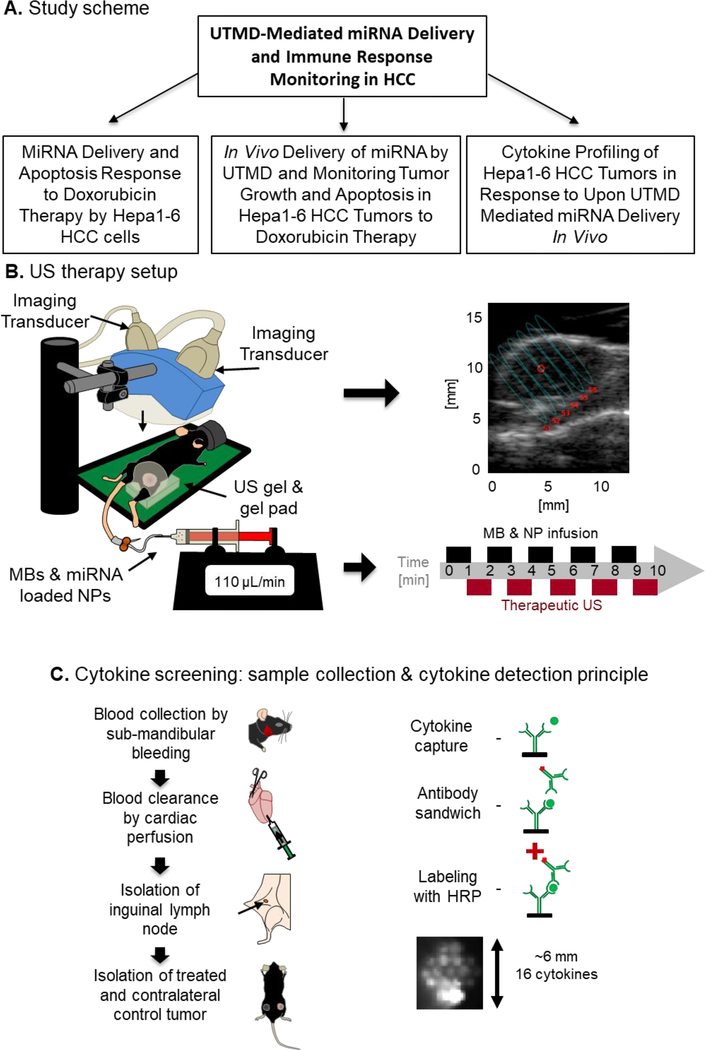
FIG. 1.
Study design. (A) Schematic workflow of the study: In order to study the effect of UTMD-mediated miRNA delivery on the immune response of HCC in vivo, the study was performed in three phases. (B) US therapy setup: The US device comprises an imaging transducer for guidance and a therapy transducer for focused mechanical US inducing MB cavitation. The US device is lowered until transducers and tissues are coupled using US gel. B-mode is used to focus the US energy on the center of the tumor. The therapy protocol lasts 10 min with alternating cycles of MB & NP infusion and cavitation. (C) Cytokine screening: Tumors were treated by UTMD-mediated delivery of miR-122/anti-miR-21-loaded PLGA-NPs. Serum was collected by submandibular bleeding. The blood pool was cleared by cardiac perfusion with PBS and inguinal lymph nodes and tumors were collected. Using a multiplex cytokine array assay, cytokines were captured with anti-cytokine antibodies, immobilized at the bottom of a 96-well plate and revealed like in a Western blot analysis.