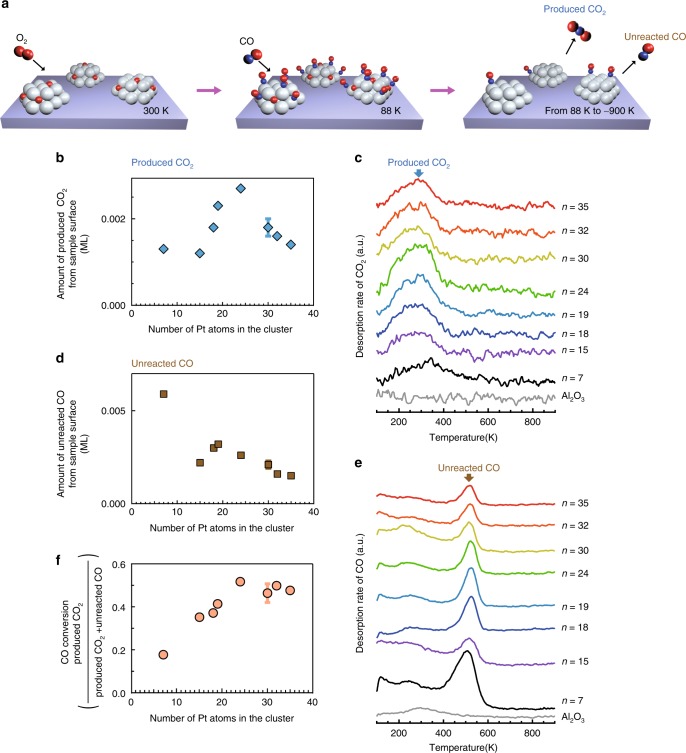
Fig. 1. CO oxidation activity of mass-selected Ptn clusters on Al2O3.
a Overview of the CO2 temperature-programmed reaction (TPR) measurements. The Ptn-deposited surfaces were exposed to 1000 L of 18O2 at 300 K to saturate the Ptn clusters with 18O atoms, followed by saturation adsorption of 13CO at 88 K, and finally the TPR measurement was performed. The Pt coverage was 0.02 ML. b Amount of produced CO2 from sample surface with mass-selected Pt single clusters relative to the number of Pt atoms in the cluster. The error bars were determined from multiple measurements on a single cluster size. c CO2 TPR spectra (m/z = 47) over Ptn/Al2O3. The number of produced CO2 molecules was estimated by integrating the intensity between 100 and 500 K. d Amount of unreacted CO molecules relative to the number of Pt atoms in the cluster. e CO TPR spectra (m/z = 29) over Ptn/Al2O3. f CO conversion (ratio of produced CO2 molecules to adsorbed CO molecules) as a function of the number of Pt atoms in the cluster. The error bars in b, d, and f are standard deviation. Source data are provided as a Source Data file.