FIG 3.
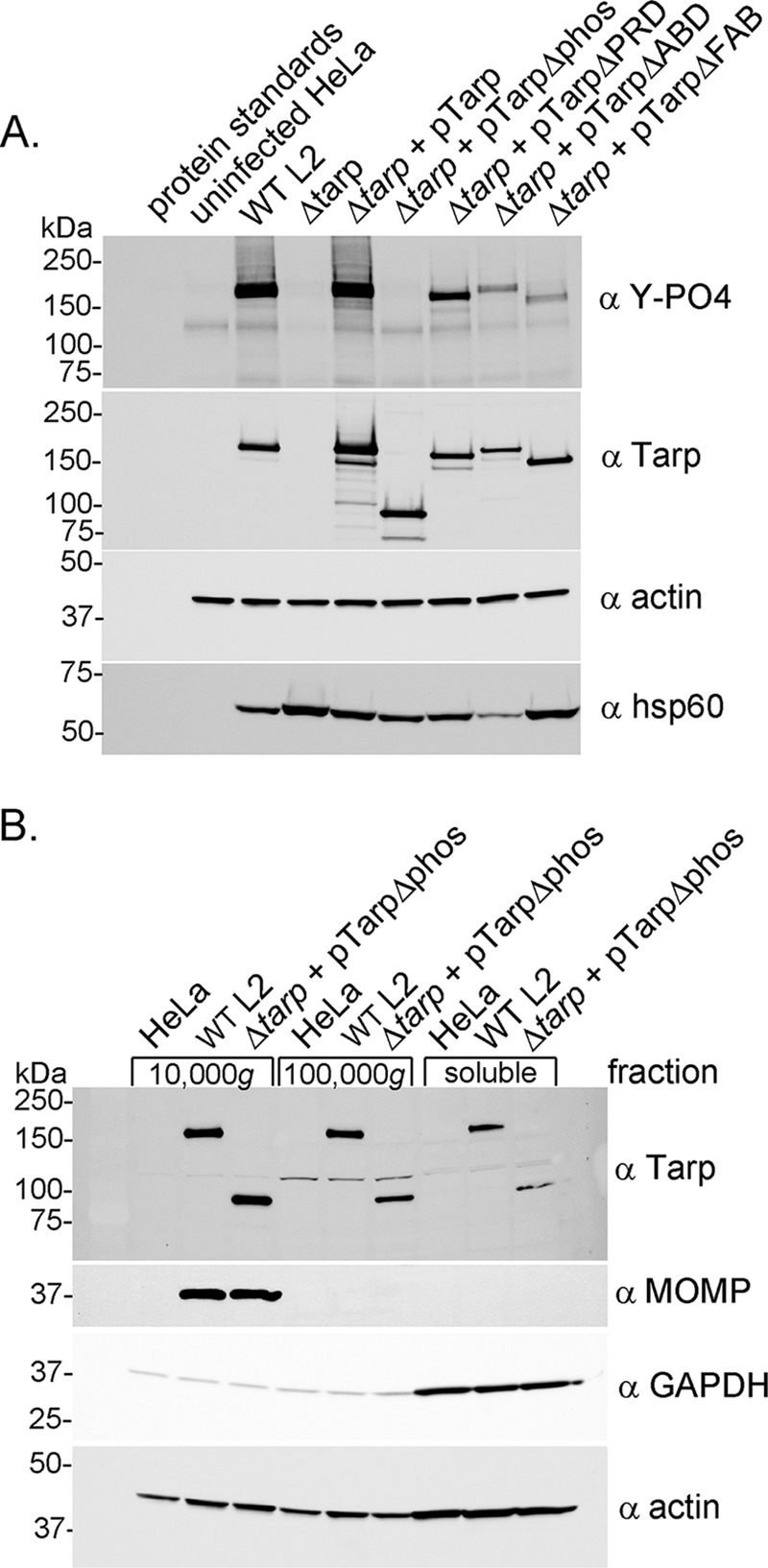
Tarp secretion and phosphorylation by Chlamydia trachomatis L2 tarP mutant and complemented clones. (A) Protein lysates were generated from HeLa cells infected with wild-type C. trachomatis L2 (WT L2), a tarP deletion mutant (Δtarp), and mutants complemented with full-length tarP (Δtarp + pTarp), tarP lacking the phosphorylation domain (Δtarp + pTarpΔphos), tarP lacking the proline-rich domain (Δtarp + pTarpΔPRD), tarP lacking the actin-binding domain (Δtarp + pTarpΔABD), or tarP lacking the F-actin-binding domains (Δtarp + pTarpΔFAB). Protein samples underwent immunoblotting analysis with phosphotyrosine (α Y-PO4), Tarp (α Tarp), actin (α actin), and C. trachomatis heat shock protein 60 (α Hsp60)-specific antibodies. A protein lysate generated from uninfected HeLa cells (uninfected HeLa) served as a negative control. (B) Subcellular fractionation of C. trachomatis-infected cells by differential centrifugation out to 100,000 × g yields a soluble Tarp fraction that is distinct from intact elementary bodies. Total lysates derived from HeLa cells alone or HeLa cells infected with wild-type C. trachomatis L2 (WT L2) or the tarP mutant complemented with pTarpΔphos (Δtarp + pTarpΔphos) underwent subcellular fractionation by centrifugation. Fractions were resolved by SDS-PAGE and transferred to nitrocellulose for immunoblotting analysis with antibodies specific for phosphotyrosine (α Y-PO4); Tarp (α Tarp); C. trachomatis major outer membrane protein (α MOMP); glyceraldehyde-3-phosphate dehydrogenase, a soluble protein marker (α GAPDH); and actin, a protein expected to be present in all fractions (α actin).
