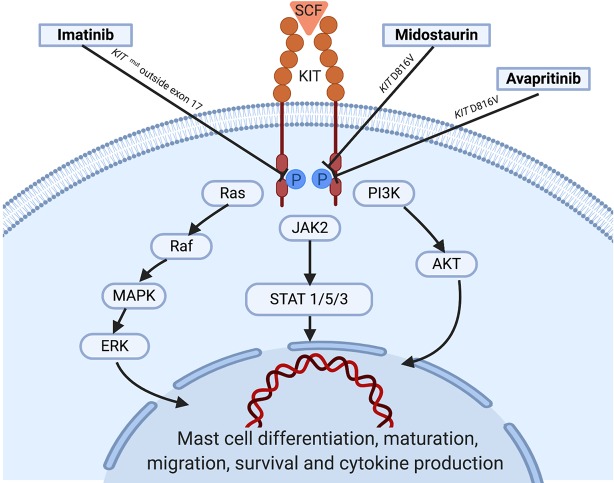
Figure 1.
KIT activation in normal mast cells: Under normal conditions, soluble SCF binds to KIT leading to receptor dimerization and kinase domain activation, which induces the initiation of a cascade of multimolecular phosphorylation events involving a variety of intracellular signal transduction pathways such as the phosphatydylinositol triphosphate kinase (PI3K) pathway, the Janus kinase (JAK) / signal transducers and activators of transcription (STAT) pathway, and the rat sarcoma (Ras)/extracellular signal-regulated kinases (ERK) pathway (Orfao et al., 2007; Cruse et al., 2014; Grinfeld et al., 2018), among others. In parallel with the complex process underlying KIT activation, strict regulatory mechanisms including the monoubiquitination of KIT that occurs after KIT/SCF binding and the action of inhibitory molecules such as SHP-1, PKC, or SOCS-1 play an important role in hampering exaggerated and potentially harmful activation states of the receptor. Information about the targets of the tyrosine kinase inhibitors showing activity is illustrated. SCF, stem cell factor; WT, wild type.