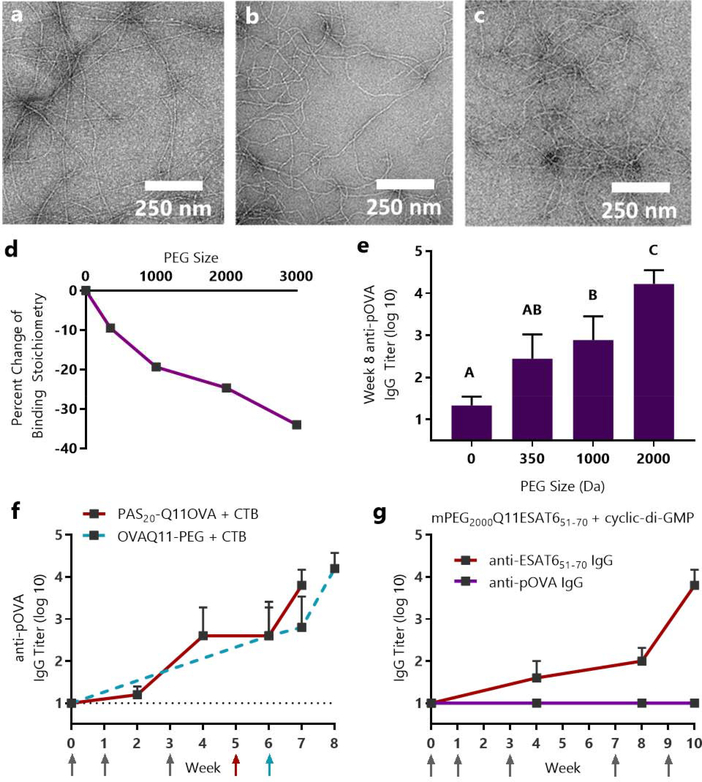
Figure 5. Sublingual immune responses and mucin binding to OVAQ11 were dependent on the length of conjugated PEG.
Q11OVA with N-terminal mPEG blocks of 350, 1000, and 2000 average molecular weight formed nanofibers, as evidenced by negative stained TEM: (a) mPEG350-Q11OVA (b) mPEG1000-Q11OVA (c) mPEG2000Q11OVA. (d) Isothermal titration calorimetry indicated that increasing PEG length diminished interactions with mucin. * p < 0.05, R2 = 0.94 by linear regression of PEG size vs. percent change in binding stoichiometry. (e) Increasing PEG length improved OVA-specific titers raised against OVAQ11-PEG nanofibers. Mice were immunized sublingually with 7 μL of 5 mM peptide with 2 μg of cholera toxin per mouse and boosted with the same dose at weeks 1, 3, and 6. IgG against the pOVA epitope was measured at week 8 by ELISA. A, B, C: Groups in (e) that do not share a letter are statistically different (p < 0.05) by 2-way ANOVA with Tukey’s multiple comparisons test, n=6 (0 PEG) or n=8/group from two independent experiments. Complete titer data is shown in Fig. S11. (f) Proline-Alanine-Serine modification (PASylation) had a similar effect as PEGylation, enabling sublingual immunization against PAS20-Q11OVA nanofibers in a fully peptidic formulation. Mice were immunized with 8 μL of 5 mM peptide and 10 μg CTB at weeks 0, 1, 3, and 5. Data from OVAQ11-PEG + CTB immunizations are re-presented from Figure 4f as a comparison. Arrows indicate timepoints of immunizations. (g) Sublingual immunization was achieved against the ESAT651–70 epitope from M. tuberculosis using cyclic-di-AMP adjuvant. CBA/J mice (n=5) were immunized at weeks 0, 1, 3, 7, and 9 with 8 μL of 5 mM mPEG2000-Q11ESAT651–70 with 10 μg of cyclic-di-AMP adjuvant per mouse; IgG against the ESAT651–70 and pOVA epitopes were measured by ELISA.