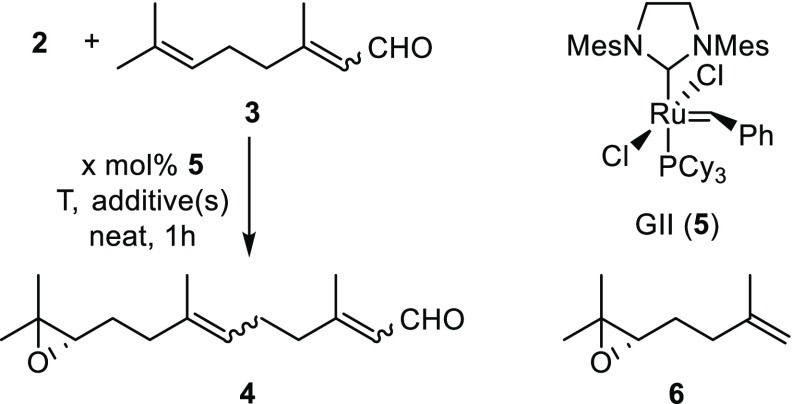
Table 1. ReXM of Relay-Actuated Δ6,7-Functionalized Monoterpenoid 2 with Citral (3) Using GII Catalyst (5)a.
| entry | equiv of 3 | mol % 5 | T (°C) | additive(s) (mol %) | % yieldb |
|---|---|---|---|---|---|
| 1 | 5 | 10 | 50 | 0c | |
| 2 | 10 | 10 | 50 | 0c | |
| 3 | 5 | 10 | RT | 0c | |
| 4 | 5 | 2 | 50 | 0c | |
| 5 | 5 | 10 | 50 | pBQ (20) | 0c |
| 6 | 5 | 10 | 50 | AcOH (20) | 64 |
| 7 | 5 | 10 | 70 | AcOH (20) | 19 |
| 8 | 5 | 10 | RT | AcOH (20) | 0c |
| 9 | 10 | 10 | 50 | AcOH (20) | 30 |
| 10 | 5 | 2 | 50 | AcOH (20) | 4 |
| 11 | 5 | 10 | 50 | AcOH (20), CuI (15) | 68 |
| 12 | 5 | 20 | 50 | AcOH (20) | 80 |
| 13 | 5 | 20 | 50 | AcOH (20), CuI (30) | 84 |
| 14 | 5 | 20 | 50 | AcOH (40), CuI (30) | 88 |
| 15 | 5 | 10d | 50 | AcOH (20) | 14 |
Reactions conducted on a 0.25 mmol scale.
Isolated yields of sesquiterpene 4 after chromatography; E/Z ratio determined as ca. 3:1 at the newly formed olefin (Δ6,7) and as ca. 2–3:1 at the α,β-unsaturated aldehyde by 1H NMR and assigned on the basis of characteristic 13C NMR shielded methyl resonances for E-isomers (see the Supporting Information).
Purification not attempted due to complex mixtures of products.
Hoveyda–Grubbs II catalyst employed.