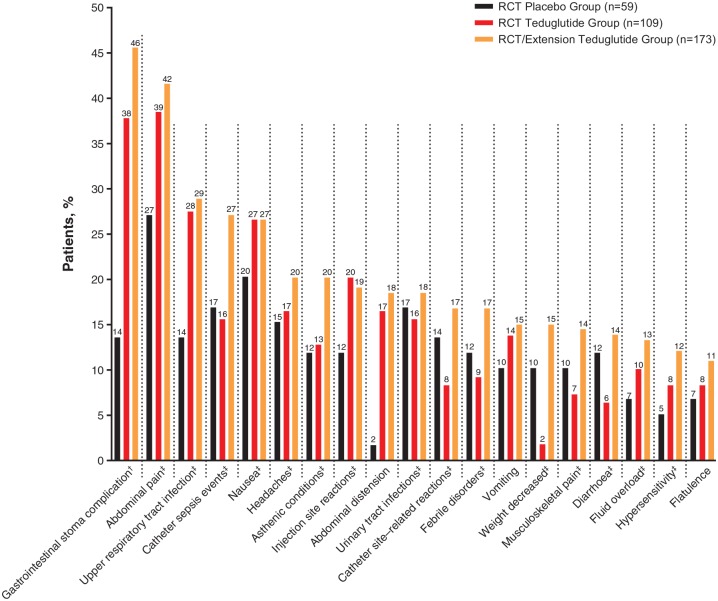
Figure 2.
Frequency of TEAEs reported in at least 10.0% of patients in the RCT/extension teduglutide group.
†Percentages calculated based on number of patients with a stoma (n = 45 for the RCT teduglutide group; n = 68 for the RCT/extension teduglutide group; n = 22 for the RCT placebo group).
‡AE grouping; the preferred terms in the AE groupings represent medically similar terms.
AE, adverse event; RCT, randomized controlled trial; TEAE, treatment-emergent AE.