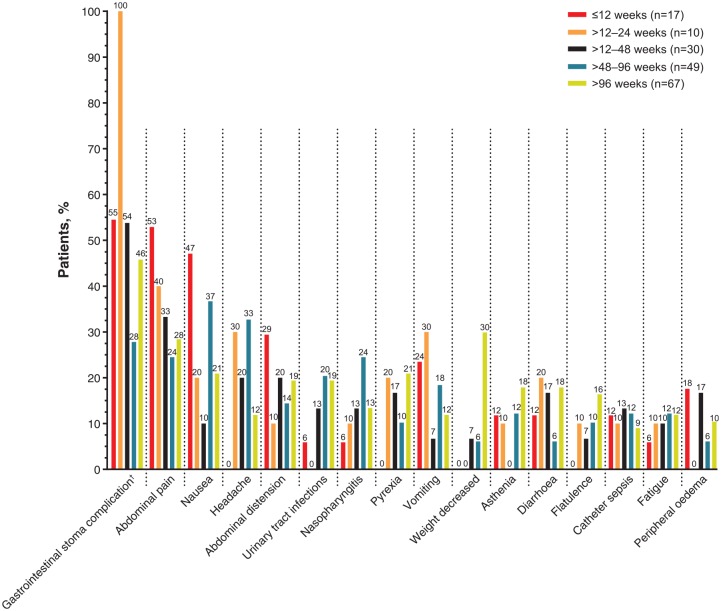
Figure 3.
Frequency of TEAEs reported in at least 10.0% of patients in the RCT/extension teduglutide group by treatment duration.
†Percentages calculated based on number of patients with a stoma (n = 11 for ⩽12 weeks; n = 2 for >12–24 weeks; n = 13 for >24–48 weeks; n = 18 for >48–96 weeks; n = 24 for >96 weeks).
RCT, randomized controlled trial; TEAE, treatment-emergent adverse event.