Fig. 4.
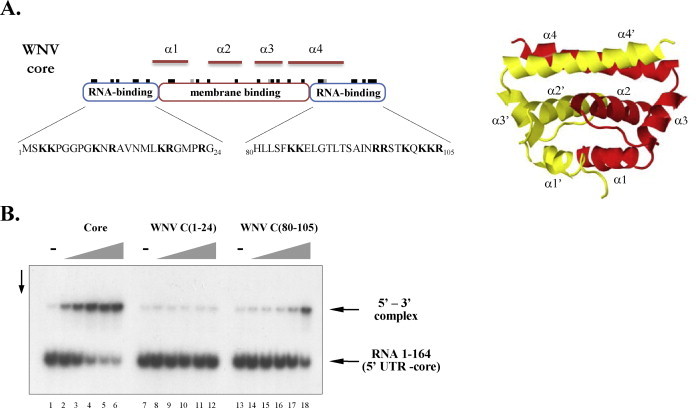
The C-terminal RNA-binding region of core directs RNA annealing. (A) Domain organization and structure of WNV core protein. The position of basic and acidic amino acids is illustrated by black and grey notches, respectively, on top of the domain outlines. The sequence of WNV C(1–24) and WNV C(80–105) peptides is indicated below the RNA-binding domains. The 3D structure of the WNV core dimer, determined by X-ray crystallography, is shown to the right (based on PDB entry 1SFK (Dokland et al., 2004), rendered by Jmol). (B) Radioactively labelled RNA 1–164 was incubated with 3′FL-UTR RNA in the presence of increasing concentrations of full-length WNV core (lanes 2–6), WNV C(1–24) (lanes 8–12), or WNV C(80–105) (lanes 14–18). Protein-to-nucleotide molar ratios were 1/40 (lanes 2, 8, 14), 1/20 (lanes 3, 9, 15), 1/10 (lanes 4, 10, 16), 1/5 (lanes 5, 11, 17), and 1/2.5 (lanes 6, 12, 18). Lanes 1, 7, and 13 show RNA annealing in the absence of core protein.
