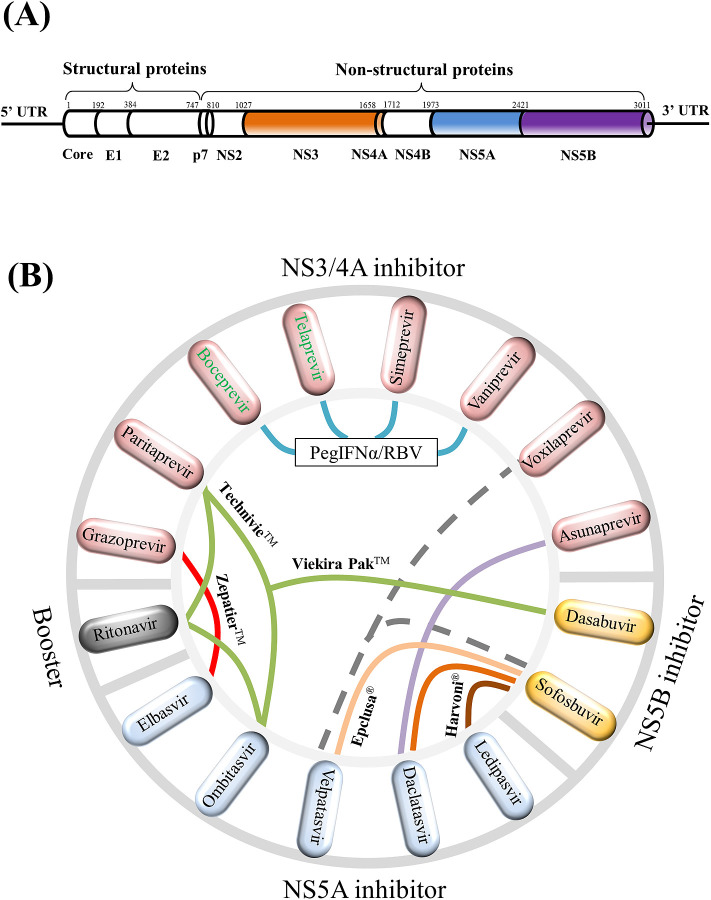
Fig. 1.
HCV genome structure and schematic view of HCV combination drugs. (A) HCV genome structure. In the length of approximately 3011 amino acids, the HCV genome codes for three structural proteins (core, E1, E2) and seven non-structural proteins (p7, NS2, NS3, NS4A, NS4B, NS5A, NS5B) whose amino acid positions are mapped accordingly. The 5′ untranslated region (5′ UTR) and the 3′ untranslated region (3′ UTR) are also indicated. Approved antiviral agents directly target to NS3/4A, NS5A, and NS5B for effective inhibition of HCV replications. (B) A total of 15 NS3/4A, NS5A, NS5B compounds plus ritonavir are displayed in the circle. Colored links in the center visualize 13 drug combinations: (i) boceprevir (Victrelis®) + PegIFNα/RBV, (ii) telaprevir (Incivek®) + PegIFNα/RBV, (iii) sofosbuvir (Sovaldi®) + PegIFNα/RBV, (iv) simeprevir (Olysio®) + PegIFNα/RBV, (v) ledipasvir + sofosbuvir (Harvoni®), (vi) ombitasvir + paritaprevir + ritonavir + dasabuvir (Viekira Pak™), (vii) ombitasvir + paritaprevir + ritonavir (Technivie™), (viii) daclatasvir (Daklinza™) + sofosbuvir (Sovaldi®), (ix) elbasvir + grazoprevir (Zepatier™), (x) sofosbuvir + velpatasvir (Epclusa®), (xi) vaniprevir (Vanihep®) + PegIFNα/RBV, (xii) asunaprevir (Sunvepra®) + daclatasvir (Daklinza®), (xiii) voxilaprevir + velpatasvir + sofosbuvir. Notably, (i) to (x) were approved by the FDA and could be used with or without ribavirin; (xi) and (xii) were approved in Japan; and (xiii) is currently under assessment by the FDA. Two discontinued drugs boceprevir and telaprevir are indicated by green texts. This figure shows that HCV combination drugs are composed of anti-HCV inhibitors from different drug classes.