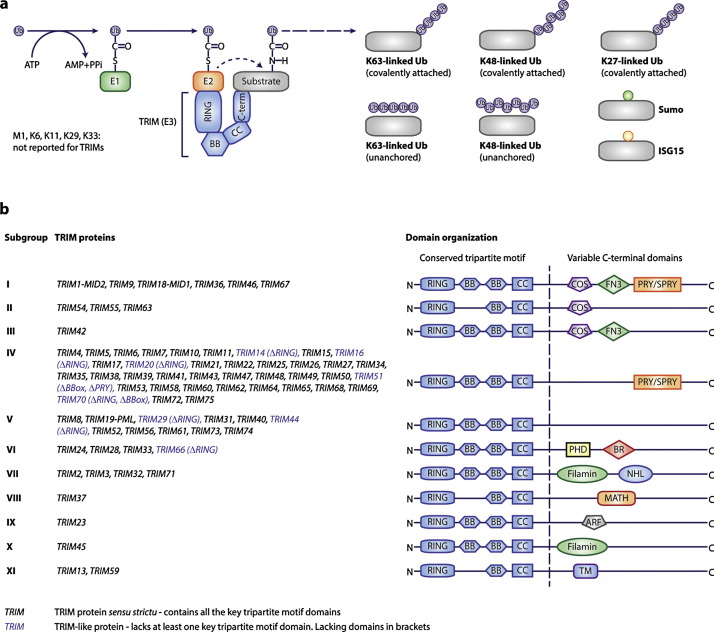
Fig. 1.
(a) Overview of ubiquitin conjugation by RING E3 ligases such as TRIM proteins. After ubiquitin activation by the E1 enzyme, ubiquitin is covalently attached to an E2 conjugase through a thio-ester bond. Subsequently, the TRIM E3 ligases bind this E2 complex through their RING domain, while determining specificity by associating with the ubiquitination target often through one of its C-terminal domains. This brings the ubiquitin-loaded E2 in close proximity to the intended target, after which the ubiquitin moiety is directly transferred to a lysine residue in the target protein by an isopeptide bond. Subsequently, additional ubiquitins can be conjugated onto internal lysines in ubiquitin or its N-terminal methionine (M1). The same ubiquitin chains can also be synthesized without being covalently coupled to their targets (unanchored ubiquitin chains). (b) Overview of the domain organization of TRIM family members.