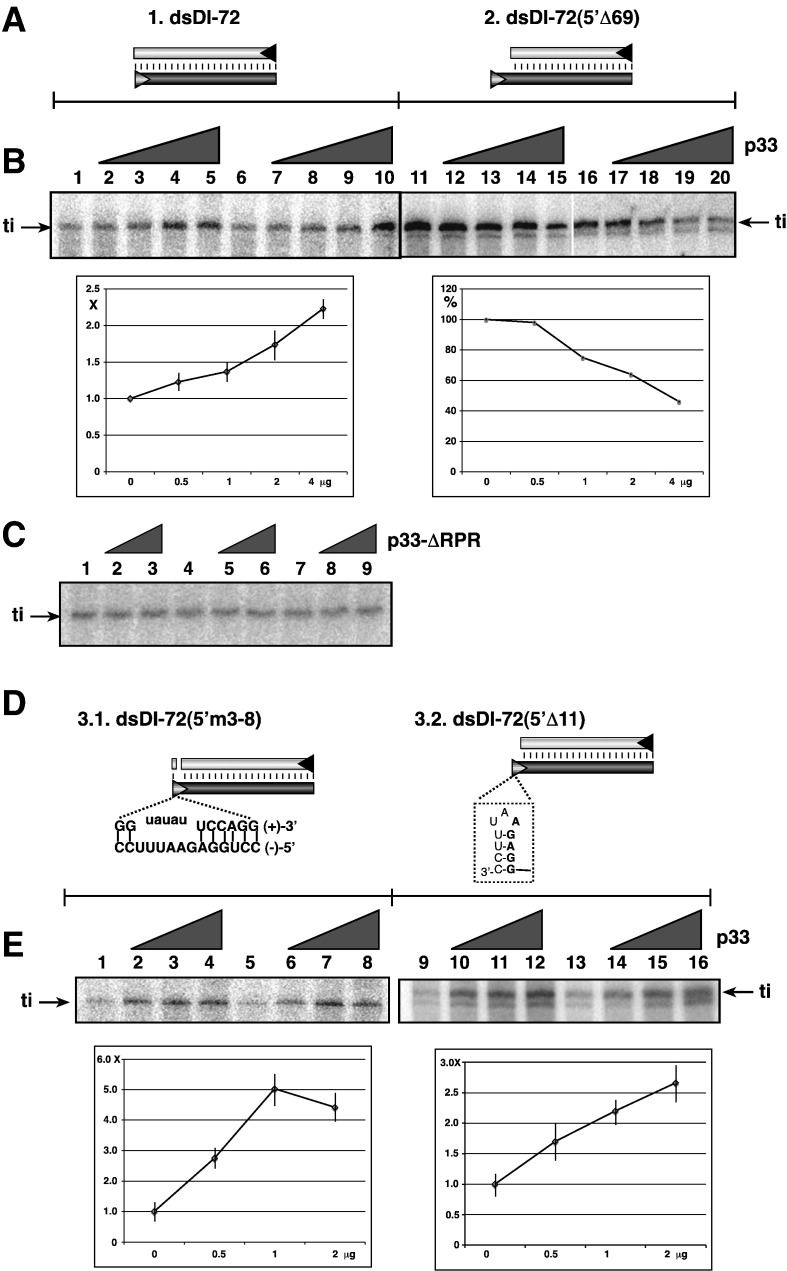
Fig. 5.
Recombinant CNV p33 promotes initiation on dsRNA template by the recombinant TCV RdRp. (A) Schematic presentation of the two dsRNA templates used to program the TCV p88C RdRp preparation in vitro. (B) Representative denaturing gels of 32P-labeled RNA products synthesized by in vitro transcription with TCV p88C RdRp in the presence of 0, 0.5, 1, 2 and 4 μg of purified recombinant p33 are shown. The dsRNA templates were gel-isolated after annealing of plus- and minus-stranded RNAs (in 1:1 ratio), and they were used in equal amounts (0.5 μg per sample). The level of RNA synthesis was compared to that of the RdRp activity obtained in the absence of p33 (100%). The samples were treated with S1 nuclease to exclude terminal transferase-based labeling of dsDI-72, which might be present in the affinity-purified TCV p88C or CNV p33 preparations. Each experiment was repeated three times. “ti” represents terminally initiated template-sized RNA product. (C) Representative denaturing gels of radiolabeled RNA products synthesized by in vitro transcription with TCV p88C RdRps in the presence of 0, 1 and 2 μg of a p33 mutant are shown. The p33 mutant lacked the RPR sequence involved in RNA-binding. See Fig. 5B for further details. Each experiment was repeated at least three times. (D) Schematic presentation of the two RNA templates used to program the TCV p88C RdRp preparation in vitro. See Fig. 5A for further details. (E) Representative denaturing gels of radiolabeled RNA products synthesized by in vitro transcription with p88C RdRp in the presence of 0, 0.5, 1 and 2 μg of purified recombinant p33 are shown. The samples were treated with S1 nuclease. See Fig. 5B for further details.